Atomic __________ is a whole number for an element.
Number
Weight
Radius
None of these
Correct Answer :
A. Number
Related Questions
The concentration of (H+) ions is 4 x 10-5 in a solution. Then pH of the solution will be (Given log 4 = 0.6)
4.4
5.6
8.4
2.4
An ideal material for making cooking vessels should have
High heat capacity
Low heat capacity
High thermal conductivity
Both (B) and (C)
The phenomenon occurring during explosion of a hydrogen bomb is
Nuclear fission
Nuclear fusion
A combination of both nuclear fission & fusion
None of these
Limestone addition in the blast furnace is done to flux __________ present in the raw materials.
SiO2
Al2O3
MnO2
P
The refrigerant freon-12 is chemically
CCl2F2
CCl3F
CClF3
CCl4F
A solar cell converts the sunlight directly into __________ energy.
Thermal
Electrical
Mechanical
Chemical
Direct conversion of thermal energy to electrical energy is facilitated by the
Fuel cells
Photo voltaic cell
Magneto hydrodynamic generator
None of these
Volumetric composition of flue gas analysed with the Orsat apparatus is : CO2 = 12%, O2 = 8%, CO = nil, N2 = 80%. This flue gas composition indicates that
Pure oxygen has been used for combustion
Nitrogen percentage in the fuel is very high
Excess air has been used for combustion
Hydrogen is not present in the fuel
Air-petrol ratio for maximum power generation in spark ignition engine is about
6 : 1
12 : 1
18 : 1
24 : 1
Increase in temperature, in general results in the
Decrease in the viscosities of both liquids & gases
Increase in the viscosities of both liquids & gases
Increase in the viscosity of liquids and decrease in that of gases
Decrease in the viscosity of liquids and increase in that of gases
At 100% relative humidity, the dew point temperature of moist air is
Less than the wet bulb temperature
More than the wet bulb temperature
Equal to the wet bulb temperature
Equal to the ambient temperature
Property responsible for the talcum powder to cling to the skin is the
Capillary action
Adhesion
Cohesion
Surface tension
Nusselt number is related to Grashoff number (Gr) in turbulent & laminar flow respectively, in respect of free convection over a vertical flat plate as
Gr0.25, Gr
Gr0.25, Gr0.33
Gr, Gr0.25
Gr0.33, Gr0.25
For efficient performance of a blast furnace, the extent of reduction of Wustite (FeO) should be
100% indirect reduction
100% direct reduction
50-60% indirect reduction
30-40% indirect reduction
Which of the following processes follows the hardening process for reducing the hardening strains & increasing the toughness of the steel part?
Anodising
Tempering
Carburising
Annealing
Heating of ferromagnetic materials to a temperature above Curie temperature makes it
Insulator for heat & electricity transmission
Ferritic
Behave like paramagnetic materials
Superconductor
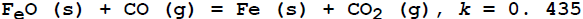 at 1173E; at equilibrium, what will be the number of moles of CO gas required to reduce one mole of
at 1173E; at equilibrium, what will be the number of moles of CO gas required to reduce one mole of 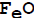 at 1173 K?
at 1173 K?
1.0
1.3
2.3
3.3
Cassiterite is an ore of
Tin
Lead
Molybdenum
Chromium
__________ can replace tungsten in high speed steel.
Chromium
Vanadium
Cobalt
Molybdenum
Specific gravity of hot metal (pig iron) is __________ times that of the blast furnace slag.
2
3
0.8
6
Specific gravity of a metal, which weighs 5 kg in air and 4 kg when submerged in water, will be
5
1.25
2.5
3.75
The thermodynamic law, 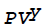 = constant, is not followed by the
= constant, is not followed by the
Free expansion of an ideal gas
Adiabatic expansion of steam in turbine
Adiabatic compression of air
Ideal compression of air
Which of the following is not a characteristic observed in material failure by fatigue fracture?
Plastic deformation of material does not occur
Initiation of crack from below the surface does not occur
Initiation of crack occurs on the surface of the machine part
Presence of both rough & smooth zone with conchoidal markings in smooth zone of the surface
Boiler draught of 10 mm water column is equivalent to
1 kgf/m2
10 kgf/m2
10 kgf/mm2
1 kgf/mm2
Increasing the carbon content of steel
Reduces the upper shelf energy
Increasing the ductility transition temperature
Decreases brittleness
Decreases hardness
Consumable electrodes are used in the __________ welding.
Gas
Arc
Thermit
Resistance
With increase in impurities in metals, their corrosion resistances
Increase
Decrease
Remain same
May increase or decrease; depending on the type of metal
Resilience of a bolt can be increased by increasing its
Length
Shank diameter
Head diameter
None of these
A fire tube boiler is limited to a maximum steam pressure of about __________ kg/cm2.
6
18
38
52
Tip of the match stick contains a mixture of
K + S
K + S + K2Cr2O7
S + K2Cr2O7 + White P
None of these
