During adiabatic expansion of gas
Pressure remains constant
Pressure is increased
Temperature remains constant
None of these
Correct Answer :
D. None of these
Related Questions
Claude gas liquefaction process employs cooling
At constant pressure
By throttling
By expansion in an engine
None of these
Gibbs free energy (F) is defined as
F = E - TS
F = H - TS
F = H + TS
F = E + TS
Henry's law is closely obeyed by a gas, when its __________ is extremely high.
Pressure
Solubility
Temperature
None of these
Pick out the correct statement:
In an isothermal system, irreversible work is more than reversible work
Under reversible conditions, the adiabatic work is less than isothermal work
Heat, work, enthalpy and entropy are all 'state functions'
Matter and energy cannot be exchanged with the surroundings in a closed system
The thermodynamic law, PVy = constant, is not applicable in case of
Ideal compression of air
Free expansion of an ideal gas
Adiabatic expansion of steam in a turbine
Adiabatic compression of a perfect gas
A system is said to be isopiestic, if there is no __________ change.
Temperature
Pressure
Volume
None of these
For equilibrium process (i.e. reversible) in an isolated system
ds = 0
ds < 0
ds > 0
ds = Constant
Heating of water under atmospheric pressure is an __________ process.
Isochoric
Isobaric
Adiabatic
Isothermal
At normal boiling point, molar entropy of vaporisation is __________ Joule/K°.mole.
72
92
142
192
Pick out the correct statement.
Entropy and enthalpy are path functions
In a closed system, the energy can be exchanged with the surrounding, while matter cannot be exchanged
All the natural processes are reversible in nature
Work is a state function
In the equation, PVn = constant, if the value of n = ± ∞, then it represents a reversible __________ process.
Adiabatic
Isometric
Isentropic
Isothermal
A gas can be liquefied by pressure alone only, when its temperature is __________ its critical temperature.
Less than
More than
Equal to or higher than
Less than or equal to
Work done in case of free expansion is
Indeterminate
Zero
Negative
None of these
The unit of fugacity is the same as that of the
Pressure
Temperature
Volume
Molar concentration
Charles' law for gases states that
V/T = Constant
V ∝ 1/T
V ∝ 1/P
PV/T = Constant
The number of degrees of freedom for a mixture of ice and water (liquid) are
3
2
1
0
A change in state involving a decrease in entropy can be spontaneous, only if
It is exothermic
It is isenthalpic
It takes place isothermally
It takes place at constant volume
The value of Cp & Cv respectively for monatomic gases in Kcal/kg Mole.°K are
5 & 3
3.987 & 1.987
1.987 & 0.66
0.66 & 1.987
PVy = constant, holds good for an isentropic process, which is
Reversible and isothermal
Isothermal and irreversible
Reversible and adiabatic
Adiabatic and irreversible
Which of the following is affected by the temperature?
Fugacity
Activity co-efficient
Free energy
All (A), (B) & (C)
Consider the process A & B shown in the figure given below: In this case, it is possible that
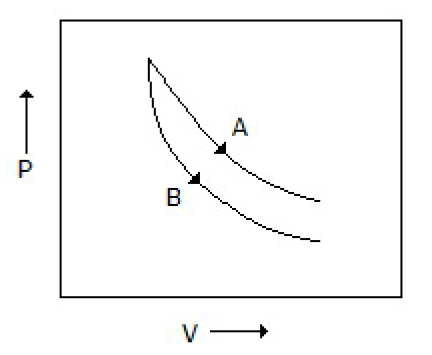
Both the processes are adiabatic
Both the processes are isothermal
Process A is isothermal while B is adiabatic
Process A is adiabatic while B is isothermal
Joule-Thomson co-efficient is defined as
μ = (∂P/∂T)H
μ = (∂T/∂P)H
μ = (∂E/∂T)H
μ = (∂E/∂P)H
Clausius-Clapeyron equation is applicable to __________ equilibrium processes.
Solid-vapor
Solid-liquid
Liquid-vapor
All (A), (B) and (C)
What is the degree of freedom for a system comprising liquid water equilibrium with its vapour?
0
1
2
3
The internal energy of an incompressible fluid depends upon its
Pressure
Temperature
Both (A) & (B)
Neither (A) nor (B)
The expression for entropy change given by, ΔS = - nR ln (P2/P1), holds good for
Expansion of a real gas
Reversible isothermal volume change
Heating of an ideal gas
Cooling of a real gas
What happens in a reversible adiabatic expansion process?
Heating takes place
Cooling takes place
Pressure is constant
Temperature is constant
Pick out the extensive property out of the following.
Surface tension
Free energy
Specific heat
Refractive index
Throttling (Joule-Thomson effect) process is a constant __________ process.
Enthalpy
Entropy
Pressure
None of these
The chemical potential of a component (μi) of a phase is the amount by which its capacity for doing all work, barring work of expansion is increased per unit amount of substance added for an infinitesimal addition at constant temperature and pressure. It is given by
(∂E/∂ni)S, v, nj
(∂G/∂ni)T, P, nj = (∂A/∂ni) T, v, nj
(∂H/∂ni)S, P, nj
All (A), (B) and (C)
