Gibbs phase rule finds application, when heat transfer occurs by
Conduction
Convection
Radiation
Condensation
Correct Answer :
D. Condensation
Related Questions
At absolute zero temperature, the __________ of the gas is zero.
Pressure
Volume
Mass
None of these
The number of degrees of freedom for a mixture of ice and water (liquid) are
3
2
1
0
After throttling, gas temperature
Decreases
Increases
Remain same
May increase or decrease; depends on the nature of the gas
In a homogeneous solution, the activity coefficient of a component depends upon the
Pressure
Composition
Temperature
All (A), (B) and (C)
Requisites of a reversible process is that the
System and surroundings pressure be equal
Friction in the system should be absent
System and surroundings temperature be equal
None of these
Pick out the wrong statement.
The values of (∂P/∂V)T and (∂2P/∂V2)T are zero for a real gas at its critical point
Heat transferred is equal to the change in the enthalpy of the system, for a constant pressure, non-flow, mechanically reversible process
Thermal efficiency of a Carnot engine depends upon the properties of the working fluid besides the source & sink temperatures
During a reversible adiabatic process, the entropy of a substance remains constant
Which is not constant for an ideal gas?
(∂P/∂V)T
(∂V/∂T)P
(∂P/∂V)V
All (A), (B) & (C)
What happens in a reversible adiabatic compression?
Heating occurs
Cooling occurs
Pressure is constant
Temperature is constant
__________ decreases during adiabatic throttling of a perfect gas.
Entropy
Temperature
Enthalpy
Pressure
Pressure-enthalpy chart is useful in refrigeration. The change in internal energy of an ideal fluid used in ideal refrigeration cycle is
Positive
Negative
Zero
Infinity
Entropy of an ideal gas depends upon its
Pressure
Temperature
Both (A) & (B)
Neither (A) nor (B)
The amount of heat required to decompose a compound into its elements is __________ the heat of formation of that compound from its elements.
Less than
More than
Same as
Not related to
When a gas is subjected to adiabatic expansion, it gets cooled due to
Decrease in velocity
Decrease in temperature
Decrease in kinetic energy
Energy spent in doing work
A liquid under pressure greater than its vapour pressure for the temperature involved is called a __________ liquid.
Sub-cooled
Saturated
Non-solidifiable
None of these
Which of the following diagrams does not represent an Otto cycle?
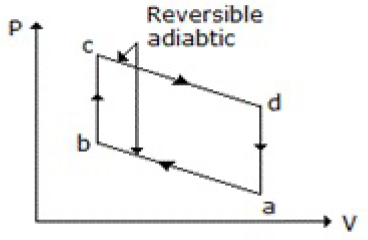
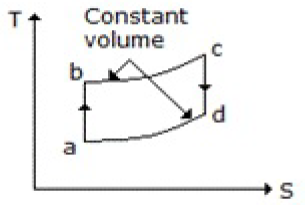
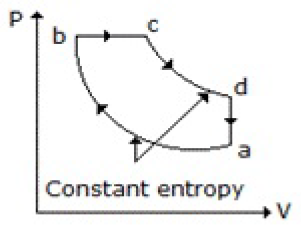
None of these
The reaction A (l) → R(g) is allowed to reach equilibrium conditions in an autoclave. At equilibrium, there are two phases, one a pure liquid phase of A and the other a vapor phase of A, R and S. Initially A alone is present. The numbers of degrees of freedom are:
1
2
3
0
Number of phases in a colloidal system is:
1
2
3
4
An isentropic process is carried out at constant
Volume
Pressure
Temperature
All (A), (B) and (C)
A chemical reaction will occur spontaneously at constant pressure and temperature, if the free energy is
Zero
Positive
Negative
None of these
Pick out the correct statement.
If an insoluble gas is passed through a volatile liquid placed in a perfectly insulated container, the temperature of the liquid will increase
A process is irreversible as long as Δ S for the system is greater than zero
The mechanical work done by a system is always equal to∫P.dV
The heat of formation of a compound is defined as the heat of reaction leading to the formation of the compound from its reactants
Water on heating from 1 to 4°C
Contracts
Expands
Has same volume
May contract or expand
Third law of thermodynamics is helpful in
Prediction of the extent of a chemical reaction
Calculating absolute entropies of substances at different temperature
Evaluating entropy changes of chemical reaction
Both (B) and (C)
Pick out the wrong statement.
An ideal liquid or solid solution is defined as one in which each component obeys Raoult's law
If Raoult's law is applied to one component of a binary mixture; Henry's law or Raoult's law is applied to the other component also
Henry's law is rigorously correct in the limit of infinite dilution
None of these
Heat of reaction at constant volume is identified with __________ change.
Enthalpy
Internal energy
Either (A) or (B)
Neither (A) nor (B)
Specific volume of an ideal gas is
Equal to its density
The reciprocal of its density
Proportional to pressure
None of these
While dissolving a gas into a liquid at a constant temperature, the ratio of the concentration of the gas in the solution phase and in the gaseous phase is
Infinity
Unity
Constant
Negative
Equation which relates pressure, volume and temperature of a gas is called the
Equation of state
Gibbs Duhem equation
Ideal gas equation
None of these
The effect of changing the evaporator temperature on COP as compared to that of changing the condenser temperature (in vapour compression refrigeration system) is
Less pronounced
More pronounced
Equal
Data insufficient, can't be predicted
First law of thermodynamics is mathematically stated as
dQ = dE + dW
dQ = dE - dW
dE = dQ + dW
dW = dQ + dE
Free energy change of mixing two liquid substances is a function of the
Concentration of the constituents only
Quantities of the constituents only
Temperature only
All (A), (B) and (C)
