Rotary lime kiln is an example of a/an __________ system.
Closed
Open
Isolated
Non-thermodynamic
Correct Answer :
B. Open
Related Questions
The intensive properties are
Molar volume, density, viscosity and boiling point
Refractive index and surface tension
Both (A) and (B)
None of these
Fugacity and pressure are numerically not equal for the gases
At low temperature and high pressure
At standard state
Both (A) and (B)
In ideal state
The kinetic energy of gas molecule is zero at
0°C
273°C
100°C
-273°C
When a gas is expanded from high pressure region to low pressure region; temperature change occurs. This phenomenon is related to the
Gibbs-Duhem equation
Gibbs-Helmholtz equation
Third law of thermodynamics
Joule-Thomson effect
Which of the following diagrams does not represent an Otto cycle?
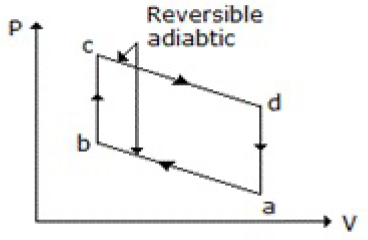
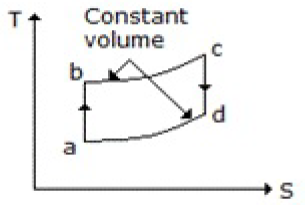
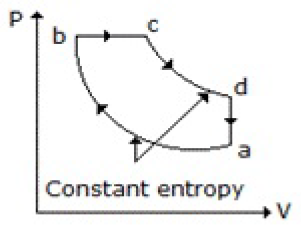
None of these
Work done may be calculated by the expression ∫ p dA for __________ processes.
Non-flow reversible
Adiabatic
Both (A) and (B)
Neither (A) nor (B)
The expression for the work done for a reversible polytropic process can be used to obtain the expression for work done for all processes, except reversible __________ process.
Isobaric
Isothermal
Adiabatic
None of these
Gibbs-Duhem equation relates composition in liquid phase and the __________ at constant temperature & pressure.
Fugacity
Partial pressure
Activity co-efficient
All (A), (B), and (C)
Gibbs free energy (G) is represented by, G = H - TS, whereas Helmholtz free energy, (A) is given by, A = E - TS. Which of the following is the Gibbs-Helmholtz equation?
[∂(G/T)/∂T] = - (H/T2)
[∂(A/T)/∂T]V = - E/T2
Both (A) and (B)
Neither (A) nor (B)
Vapour which is at a pressure smaller than the saturation pressure for the temperature involved is called a __________ vapour.
Superheated
Desuperheated
Non-condensable
None of these
Law of corresponding states says that
Two different gases behave similarly, if their reduced properties (i.e. P, V and T) are same
The surface of separation (i. e. the meniscus) between liquid and vapour phase disappears at the critical temperature
No gas can be liquefied above the critical temperature, howsoever high the pressure may be.
The molar heat of energy of gas at constant volume should be nearly constant (about 3 calories)
No work is done by the system, when a reaction occurs at constant
Volume
Temperature
Pressure
None of these
The compressibility factor for an ideal gas is 1. Its value for any other real gas is
1
< 1
> 1
Either (B) or (C), depends on the nature of the gas
Heat requirement for decomposition of a compound into its elements is __________ that is evolved during the formation of that compound from its elements.
The same
Less than
Greater than
Different than
At a given temperature, the volume of a gas dissolved in a solvent __________ with increase in pressure.
Increases
Decreases
Remains unchanged
May increase or decrease; depends on the gas
Claude's liquefaction process employs the cooling of gases by
Expansion in an engine
Following a constant pressure cycle
Throttling
None of these
On opening the door of an operating refrigerator kept in a closed room, the temperature of the room will
Increase
Decrease
Remain same
Increase in summer and will decrease in winter
The minimum number of phases that can exist in a system is
0
1
2
3
Pick out the wrong statement pertaining to the decomposition of PCl5 represented by, PCl5 PCl3 + Cl2.Degree of dissociation of PCl5 will
Decrease on addition of Cl2
Increase on addition of an inert gas at constant pressure
Decrease on increasing the pressure of the system
None of these
At the absolute zero temperature, the entropy of every perfectly crystalline substance becomes zero. This follows from the
Third law of thermodynamics
Second law of thermodynamics
Nernst heat theorem
Maxwell's relations
At constant temperature and pressure, for one mole of a pure substance, the ratio of the free energy to the chemical potential is
Zero
One
Infinity
Negative
In polytropic process (PVn = constant), if n = 1; it means a/an __________ process.
Adiabatic
Reversible
Isothermal
None of these
Which of the following is not an intensive property?
Volume
Density
Temperature
Pressure
A liquid under pressure greater than its vapour pressure for the temperature involved is called a __________ liquid.
Sub-cooled
Saturated
Non-solidifiable
None of these
In vapour compression refrigeration system, if the evaporator temperature and the condenser temperatures are -13°C and 37°C respectively, the Carnot COP will be
5.2
6.2
0.168
Data insufficient, can't be found out
Which of the following is not correct for a reversible adiabatic process?
TVγ-1 = constant
p1-γ.TY = constant
PVγ = constant
None of these
As the time is passing, entropy of the universe
Is increasing
Is decreasing
Remain constant
Data insufficient, can't be predicted
Two substances are in equilibrium in a reversible chemical reaction. If the concentration of each substance is doubled, then the value of the equilibrium constant will be
Same
Doubled
Halved
One fourth of its original value
A cyclic engine exchanges heat with two reservoirs maintained at 100 and 300°C respectively. The maximum work (in J) that can be obtained from 1000 J of heat extracted from the hot reservoir is
349
651
667
1000
The free energy change for a chemical reaction is given by (where, K = equilibrium constant)
RT ln K
-RT ln K
-R ln K
T ln K
