The area under the temperature-entropy curve (T - s curve) of any thermodynamic process represents
Heat absorbed
Heat rejected
Either (A) or (B)
None of these
Correct Answer :
C. Either (A) or (B)
Related Questions
When a beam is subjected to a bending moment, the strain in a layer is __________ the distance from the neutral axis.
Equal to
Directly proportional to
Inversely proportional to
Independent of
The maximum shear stress, in the given figure, is equal to __________ of the Mohr's circle.
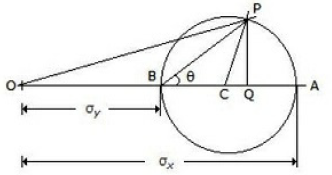
Radius
Diameter
Circumference
Area
The safe twisting moment for a compound shaft is equal to the
Maximum calculated value
Minimum calculated value
Mean value
Extreme value
Otto cycle efficiency is higher than Diesel cycle efficiency for the same compression ratio and heat input because in Otto cycle
Combustion is at constant volume
Expansion and compression are isentropic
Maximum temperature is higher
Heat rejection is lower
For the same compression ratio, the efficiency of dual combustion cycle is
Greater than Diesel cycle and less than Otto cycle
Less than Diesel cycle and greater than Otto cycle
Greater than Diesel cycle
Less than Diesel cycle
The specific heat at constant volume is
The amount of heat required to raise the temperature of unit mass of gas through one degree, at constant pressure
The amount of heat required to raise the temperature of unit mass of gas through one degree, at constant volume
The amount of heat required to raise the temperature of 1 kg of water through one degree
Any one of the above
Which of the following is a reversible non-flow process?
Isochoric process
Isobaric process
Hyperbolic process
All of these
Elasticity of Mild Steel specimen is defined by
Hookes law
Yield point
Plastic flow
Proof stress
The shape of cantilever for uniformly distributed load will be
Straight line
Parabolic
Elliptical
Cubic
The extremeties of any diameter on Mohr's circle represent
Principal stresses
Normal stresses on planes at 45°
Shear stresses on planes at 45°
Normal and shear stresses on a plane
The materials having same elastic properties in all directions are called
Ideal materials
Uniform materials
Isotropic materials
Piratical materials
When wood is heated with a limited supply of air to a temperature not less than 280°C, the resulting fuel is
Coke
Wood charcoal
Bituminous coal
Briquetted coal
When a system changes its state from one equilibrium state to another equilibrium state, then the path of successive states through which the system has passed, is known as
Thermodynamic law
Thermodynamic process
Thermodynamic cycle
None of these
The energy absorbed in a body, when it is strained within the elastic limits, is known as
Strain energy
Resilience
Proof resilience
Modulus of resilience
Which of the following materials is most elastic?
Rubber
Plastic
Brass
Steel
Flow stress corresponds to
Fluids in motion
Breaking point
Plastic deformation of solids
Rupture stress
For a beam, as shown in the below figure, the deflection at C is (where E = Young's modulus for the beam material, and I = Moment of inertia of the beam section.
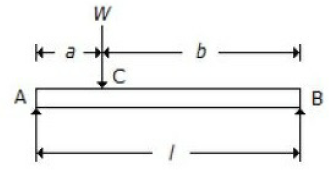
Wl3/48 EI
Wa²b²/3EIl
[Wa/(a√3) x EIl] x (l² - a²)3/2
5Wl3/384 EI
All perfect gases change in volume by 1/273th of its original volume at 0°C for every 1°C change in temperature, when the pressure remains constant. This statement is called
Boyle's law
Charles' law
Gay-Lussac law
Joule's law
The natural petroleum may be separated into
Petrol
Kerosene
Fuel oil
Lubricating oil
In a free expansion process,
W1 - 2 = 0
Q1 - 2 = 0
dU = 0
All of these
The heat energy stored in the gas and used for raising the temperature of the gas is known as
External energy
Internal energy
Kinetic energy
Molecular energy
When a gas is heated at constant volume
Its temperature will increase
Its pressure will increase
Both temperature and pressure will increase
Neither temperature nor pressure will increase
In a steady flow process, the ratio of
Heat transfer is constant
Work transfer is constant
Mass flow at inlet and outlet is same
All of these
One Joule (J) is equal to
1 N-m
1 kN-m
10 N-m/s
10 kN-m/s
A circular shaft fixed at, A has diameter D for half of its length and diameter D/2 over the other half, as shown in the below figure. If the rotation of B relative to A is 0.1 radian, the rotation of C relative to B will be
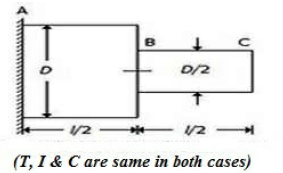
0.4 radian
0.8 radian
1.6 radian
3.2 radian
The maximum stress produced in a bar of tapering section is at
Smaller end
Larger end
Middle
Anywhere
In S. I. units, the value of the universal gas constant is
8.314 J/kg mole-K
83.14 J/kgmole-K
831.4 J/kgmole-K
8314 J/kgmole-K
The behaviour of a perfect gas, undergoing any change in the variables which control physical properties, is governed by
Boyle's law
Charles' law
Gay-Lussac law
All of these
Which of the following is the extensive property of a thermodynamic system?
Pressure
Volume
Temperature
Density
Resilience is the
Energy stored in a body when strained within elastic limits
Energy stored in a body when strained up to the breaking of the specimen maximum strain
Energy which can be stored in a body
None of the above
