The change in Gibbs free energy for vaporisation of a pure substance is
Positive
Negative
Zero
May be positive or negative
Correct Answer :
C. Zero
Related Questions
Gibbs-Duhem equation relates composition in liquid phase and the __________ at constant temperature & pressure.
Fugacity
Partial pressure
Activity co-efficient
All (A), (B), and (C)
In the ammonia synthesis reaction, N2 + 3H2 2NH3 + 22.4 kcal, the formation of NH3 will be favoured by
High temperature
Low pressure
Low temperature only
Both low temperature and high pressure
A thermodynamic system is taken from state A to B along ACB and is brought back to A along BDA as shown below in the P-V diagram. The net work done during the complete cycle is given by the area covered by
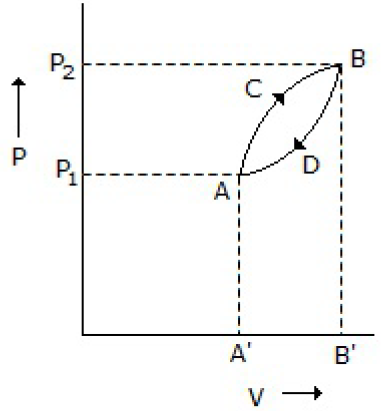
P1ACBP2P1
ACBB1A1A
ACBDA
ADBB1A1A
Partial molar free energy of an element A in solution is same as its
Chemical potential
Activity
Fugacity
Activity co-efficient
For an ideal gas, the internal energy depends upon its __________ only.
Molecular size
Temperature
Volume
Pressure
In the reaction; N2 + O2 2NO, increasing the pressure will result in
Shifting the equilibrium towards right
Shifting the equilibrium towards left
No change in equilibrium condition
None of these
No work is done by the system, when a reaction occurs at constant
Volume
Temperature
Pressure
None of these
At absolute zero temperature, the __________ of the gas is zero.
Pressure
Volume
Mass
None of these
Pick out the correct statement.
If an insoluble gas is passed through a volatile liquid placed in a perfectly insulated container, the temperature of the liquid will increase
A process is irreversible as long as Δ S for the system is greater than zero
The mechanical work done by a system is always equal to∫P.dV
The heat of formation of a compound is defined as the heat of reaction leading to the formation of the compound from its reactants
If an ideal solution is formed by mixing two pure liquids in any proportion, then the __________ of mixing is zero
Enthalpy
Volume
Both 'a' & 'b'
Neither 'a' nor 'b'
The expression, nRT ln(P1/P2), is for the __________of an ideal gas.
Compressibility
Work done under adiabatic condition
Work done under isothermal condition
Co-efficient of thermal expansion
Which of the following is a widely used refrigerant in vapour compression refrigeration system (using large centrifugal compressor)?
Freon
Liquid sulphur dioxide
Methyl chloride
Ammonia
For an ideal solution, the value of activity co-efficient is
0
1
< 1
> 1
Pick out the correct statement.
Compression ratio of an Otto engine is comparatively higher than a diesel engine
Efficiency of an Otto engine is higher than that of a diesel engine for the same compression ratio
Otto engine efficiency decreases with the rise in compression ratio, due to decrease in work produced per quantity of heat
Diesel engine normally operates at lower compression ratio than an Otto engine for an equal output of work
The principle applied in liquefaction of gases is
Adiabatic expansion
Joule-Thomson effect
Both (A) and (B)
Neither (A) nor (B)
The temperature at which a real gas obeys the ideal gas laws over a wide range of pressure is called __________ temperature.
Boyle
Inversion
Critical
Reduced
Maximum work that could be secured by expanding the gas over a given pressure range is the __________ work.
Isothermal
Adiabatic
Isentropic
None of these
What is the value of ln y (where y = activity co-efficient) for ideal gases?
Zero
Unity
Infinity
Negative
The Carnot co-efficient of performance (COP) of a domestic air conditioner compared to a household refrigerator is
Less
More
Same
Dependent on climatic conditions
Pick out the extensive property out of the following.
Surface tension
Free energy
Specific heat
Refractive index
Which of the following is true for Virial equation of state?
Virial co-efficients are universal constants
Virial co-efficients 'B' represents three body interactions
Virial co-efficients are function of temperature only
For some gases, Virial equations and ideal gas equations are the same
Which of the following is not correct for a reversible adiabatic process?
TVγ-1 = constant
p1-γ.TY = constant
PVγ = constant
None of these
Pick out the wrong statement.
The net change in entropy in any reversible cycle is always zero
The entropy of the system as a whole in an irreversible process increases
The entropy of the universe tends to a maximum
The entropy of a substance does not remain constant during a reversible adiabatic change
Keeping the pressure constant, to double the volume of a given mass of an ideal gas at 27°C, the temperature should be raised to __________ °C.
270
327
300
540
Mollier chart is a __________ plot.
Pressure vs. enthalpy
Pressure vs. volume
Enthalpy vs. entropy
Temperature vs. entropy
A large iceberg melts at the base, but not at the top, because of the reason that
Ice at the base contains impurities which lowers its melting point
Due to the high pressure at the base, its melting point reduces
The iceberg remains in a warmer condition at the base
All (A), (B) and (C)
Joule-Thomson Co-efficient at any point on the inversion curve is
∞
+ve
0
-ve
Joule-Thomson experiment is
Isobaric
Adiabatic
Isenthalpic
Both (B) & (C)
A solid metallic block weighing 5 kg has an initial temperature of 500°C. 40 kg of water initially at 25°C is contained in a perfectly insulated tank. The metallic block is brought into contact with water. Both of them come to equilibrium. Specific heat of block material is 0.4 kJ.kg-1. K-1. Ignoring the effect of expansion and contraction and also the heat capacity to tank, the total entropy change in kJ.kg-1, K-1 is
-1.87
0
1.26
3.91
Free energy change at equilibrium is
Zero
Positive
Negative
Indeterminate
