The free energy change for a chemical reaction is given by (where, K = equilibrium constant)
RT ln K
-RT ln K
-R ln K
T ln K
Correct Answer :
B. -RT ln K
Related Questions
Melting of ice is an example of an __________ process.
Adiabatic
Isothermal
Isometric
None of these
Joule-Thomson co-efficient which is defined as, η = (∂T/∂P)H = 1/Cp (∂H/∂T)P, changes sign at a temperature known as inversion temperature. The value of Joule-Thomson co-efficient at inversion temperature is
0
∞
+ve
-ve
For the reversible exothermic reaction, N2 + 3H2 2NH3, increase of pressure would
Shift the equilibrium towards right
Give higher yield of NH3
Both (B) and (C)
Neither (A) nor (B)
Change of heat content when one mole of compound is burnt in oxygen at constant pressure is called the
Calorific value
Heat of reaction
Heat of combustion
Heat of formation
Gibbs free energy (G) is represented by, G = H - TS, whereas Helmholtz free energy, (A) is given by, A = E - TS. Which of the following is the Gibbs-Helmholtz equation?
[∂(G/T)/∂T] = - (H/T2)
[∂(A/T)/∂T]V = - E/T2
Both (A) and (B)
Neither (A) nor (B)
Free energy change of mixing two liquid substances is a function of the
Concentration of the constituents only
Quantities of the constituents only
Temperature only
All (A), (B) and (C)
Gibbs-Duhem equation relates composition in liquid phase and the __________ at constant temperature & pressure.
Fugacity
Partial pressure
Activity co-efficient
All (A), (B), and (C)
In a reversible process
Tds = dE + dW
dE - dW = Tds
dW - dE = Tds
Tds - dW + dE >0
The first law of thermodynamics is a restatement of the law of conservation of
Mass
Energy
Momentum
None of these
Charles' law for gases states that
V/T = Constant
V ∝ 1/T
V ∝ 1/P
PV/T = Constant
At absolute zero temperature, the __________ of the gas is zero.
Pressure
Volume
Mass
None of these
The equation, PV = nRT, is best obeyed by gases at
Low pressure & high temperature
High pressure & low temperature
Low pressure & low temperature
None of these
One mole of nitrogen at 8 bar and 600 K is contained in a piston-cylinder arrangement. It is brought to 1 bar isothermally against a resisting pressure of 1 bar. The work done (in Joules) by the gas is
30554
10373
4988.4
4364.9
With increase in pressure (above atmospheric pressure), the Cp of a gas
Increases
Decreases
Remains unchanged
First decreases and then increases
Tea kept in a thermos flask is vigorously shaken. If the tea is considered as a system, then its temperature will
Increase
Decrease
Remain unchanged
First fall and then rise
In case of the decomposition of hydroiodic acid (2HI H2 + I2), addition of H2 (at equilibrium condition) will
Increase the partial pressure of I2
Decrease the partial pressure of HI
Diminish the degree of dissociation of HI
None of these
The extensive properties are
Volume, mass and number of moles
Free energy, entropy and enthalpy
Both (A) and (B)
None of these
Heat of reaction at constant volume is identified with __________ change.
Enthalpy
Internal energy
Either (A) or (B)
Neither (A) nor (B)
The standard Gibbs free energy change of a reaction depends on the equilibrium
Pressure
Temperature
Composition
All (A), (B) and (C)
If the pressure on 100 c.c. of air is halved, then its volume (at the same temperature) would be __________ c.c.
100
50
205
200
Ideal refrigeration cycle is
Same as Carnot cycle
Same as reverse Carnot cycle
Dependent on the refrigerant's properties
The least efficient of all refrigeration processes
Which of the following is true for Virial equation of state?
Virial co-efficients are universal constants
Virial co-efficients 'B' represents three body interactions
Virial co-efficients are function of temperature only
For some gases, Virial equations and ideal gas equations are the same
If atmospheric temperature and dew point are nearly equal, then the relative humidity is
Zero
50%
Almost 100%
unpredictable
Variation of equilibrium pressure with temperature for any two phases of a given substances is given by the __________ equation.
Gibbs-Duhem
Maxwell's
Clapeyron
None of these
Generation of heat by friction is an example of a/an __________ change.
Isothermal
Irreversible
Adiabatic
Reversible
The amount of heat required to decompose a compound into its elements is __________ the heat of formation of that compound from its elements.
Less than
More than
Same as
Not related to
Claude gas liquefaction process employs cooling
At constant pressure
By throttling
By expansion in an engine
None of these
An ideal monatomic gas is taken round the cycle ABCDA as shown below in the P-V diagram. The work done during the cycle is
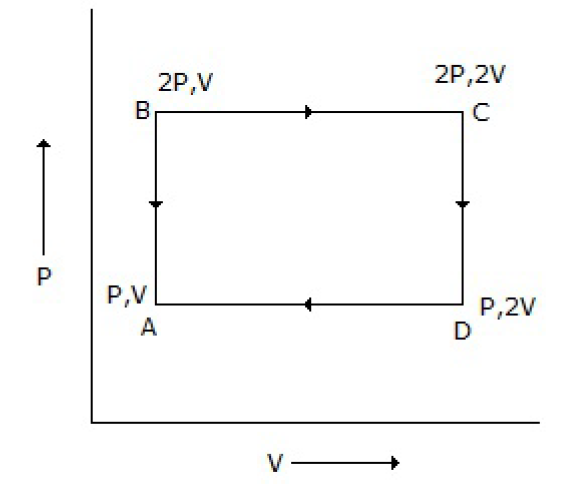
PV
2PV
PV/2
0
A domestic refrigerator has a/an __________ cooled condenser.
Water
Air
Evaporative
Gas
Standard temperature and pressure (S.T.P.) is
0°C and 750 mm Hg
15°C and 750 mm Hg
0°C and 1 kgf/cm2
15°C and 1 kgf/cm2
