The intensive properties are
Molar volume, density, viscosity and boiling point
Refractive index and surface tension
Both (A) and (B)
None of these
Correct Answer :
C. Both (A) and (B)
Related Questions
The free energy change for a chemical reaction is given by (where, K = equilibrium constant)
RT ln K
-RT ln K
-R ln K
T ln K
Which of the following identities can be most easily used to verify steam table data for superheated steam?
(∂T/∂V)S = (∂p/∂S)V
(∂T/∂P)S = (∂V/∂S)P
(∂P/∂T)V = (∂S/∂V)T
(∂V/∂T)P = -(∂S/∂P)T
Pick out the correct statement.
If an insoluble gas is passed through a volatile liquid placed in a perfectly insulated container, the temperature of the liquid will increase
A process is irreversible as long as Δ S for the system is greater than zero
The mechanical work done by a system is always equal to∫P.dV
The heat of formation of a compound is defined as the heat of reaction leading to the formation of the compound from its reactants
The co-efficient of performance (COP) of a refrigerating system, which is its index of performance, is defined as the ratio of useful refrigeration to the net work. The units of __________ and COP are the same.
Kinematic viscosity
Work
Temperature
None of these
The equation, PV = nRT, is best obeyed by gases at
Low pressure & high temperature
High pressure & low temperature
Low pressure & low temperature
None of these
In an isothermal process on an ideal gas, the pressure increases by 0.5 percent. The volume decreases by about __________ percent.
0.25
0.5
0.75
1
Which is not constant for an ideal gas?
(∂P/∂V)T
(∂V/∂T)P
(∂P/∂V)V
All (A), (B) & (C)
For water at 300°C, it has a vapour pressure 8592.7 kPa and fugacity 6738.9 kPa Under these conditions, one mole of water in liquid phase has a volume of 25.28 cm3 and that in vapour phase in 391.1 cm3.Fugacity of water (in kPa) at 9000 kPa will be
6738.9
6753.5
7058.3
9000
Melting of ice exemplifies a/an
Adiabatic process
Endothermic reaction
Exothermic reaction
Process involving a chemical reaction
Fugacity is most helpful in
Representing actual behaviour of real gases
Representing actual behaviour of ideal gases
The study of chemical equilibria involving gases at atmospheric pressure
None of these
Van Laar equation deals with the activity coefficients in
Binary solutions
Ternary solutions
Azeotropic mixture only
None of these
For an isothermal reversible compression of an ideal gas
Only ΔE = 0
Only ΔH =0
ΔE = ΔH = 0
dQ = dE
High __________ is an undesirable property for a good refrigerant.
Specific heat
Latent heat of vaporisation
Viscosity
Specific vapor volume
Sound waves propagation in air exemplifies an __________ process.
Adiabatic
Isothermal
Isometric
None of these
The partial molar enthalpy of a component in an ideal binary gas mixture of composition Z, at a temperature T and pressure P, is a function only of
T
T and P
T, P and Z
T and Z
Entropy, which is a measure of the disorder of a system, is:
Independent of pressure
Independent of temperature
Zero at absolute zero temperature for a perfect crystalline substance
All (A), (B) & (C)
If an ideal solution is formed by mixing two pure liquids in any proportion, then the __________ of mixing is zero
Enthalpy
Volume
Both 'a' & 'b'
Neither 'a' nor 'b'
Fugacity co-efficient of a substance is the ratio of its fugacity to
Mole fraction
Activity
Pressure
Activity co-efficient
Molar heat capacity of water in equilibrium with ice at constant pressure is __________ Kcal/kg mole. °K
0
∞
50
100
The freezing point of a liquid decreases when the pressure is increased, if the liquid __________ while freezing.
Contracts
Expands
Does not change in volume
Either (A), (B) or (C)
Consider the process A & B shown in the figure given below: In this case, it is possible that
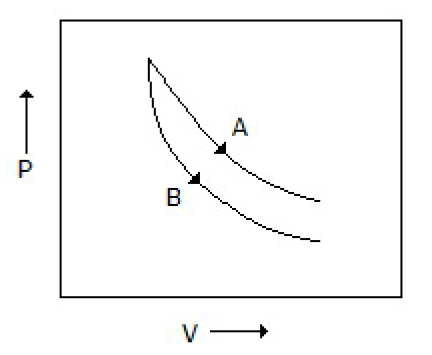
Both the processes are adiabatic
Both the processes are isothermal
Process A is isothermal while B is adiabatic
Process A is adiabatic while B is isothermal
Pick out the wrong statement.
Enthalpies of all elements in their standard states are assumed to be zero
Combustion reactions are never endothermic in nature
Heat of reaction at constant volume is equal to the change in internal energy
Clausius-Clapeyron equation is not applicable to melting process
If we increase the pressure on a substance (which is at its triple point), then the triple point
Increases
Decreases
Remains unchanged
May increase or decrease; depends on the substance
Chemical potential (an intensive property) of a substance is a force that drives the chemical system to equilibrium and is equal to its partial molar properties. The ratio of chemical potential to free energy of a pure substance at constant temperature and pressure is
0
1
∞
None of these
A Carnot cycle consists of the following steps:
Two isothermal and two isentropic
Two isobaric and two isothermal
Two isochoric and two isobaric
Two isothermals and two isochoric
It is desired to bring about a certain change in the state of a system by performing work on the system under adiabatic conditions.
The amount of work needed is path dependent
Work alone cannot bring out such a change of state
The amount of work needed is independent of path
More information is needed to conclude anything about the path dependence or otherwise of the work needed
The expression for entropy change given by, ΔS = - nR ln (P2/P1), holds good for
Expansion of a real gas
Reversible isothermal volume change
Heating of an ideal gas
Cooling of a real gas
In the equation PVn = constant, if the value of n = y = Cp/Cv, then it represents a reversible __________ process.
Isothermal
Adiabatic
Isentropic
Polytropic
If the vapour pressure at two temperatures of a solid phase in equilibrium with its liquid phase are known, then the latent heat of fusion can be calculated by the
Maxwell's equation
Clausius-Clapeyron Equation
Van Laar equation
Nernst Heat Theorem
Which of the following equations is used for the prediction of activity co-efficient from experiments?
Van Laar equation
Margules equation
Wilson's equation
All (A), (B) and (C)
