The interrelationship between matter and energy was given by
Rutherford
Joule
de Broglie
Einstein
Correct Answer :
D. Einstein
Related Questions
The orbitals with maximum number of possible orientation is:
s
d
f
p
The interrelationship between matter and energy was given by
Rutherford
Joule
de Broglie
Einstein
The fundamental particle that has least mass is
meson
alpha-particle
electron
neutron
Charge on an electron was shown by
J. J. Thomson
Kirchoff
Ohm
M.Planck
The number of unpaired electrons in a chromic ion (atomic number 24) is
6
4
3
1
The wavelength of an electron
is equal to that of light
remains constant with velocity
decreases with an increasing velocity
increases with an decreasing velocity
The charge on an atom of is
+40
+27
+14
Zero
Frequency of the wave having wave number is
The number of unpaired electrons in fluorine atoms is/are
4
2
1
0
Electronic configuration of is
The angular momentum of the moving electron is equal to
The wavelength of an electron moving with a velocity
In an atom of hydrogen, which of the following orbitais has the lowest energy for an electron present in it?
3r
2p
4p
2s
No two electrons in an atom can have the same values of all four quantum numbers according to
Hund's rules
Flemming rule
Pauli's exclusion principle
Bohr theory
A dipositive ion has in the K shell, 8 e~ s in the L shell and in the M shell. Atomic number of Z is
19
20
16
15
The number of unpaired electrons in the ground state of chromium is
1
6
7
2
The number of neutrons in the deuterium atom is
16
3
1
0
Which elements is represented by the following electronic configuration?
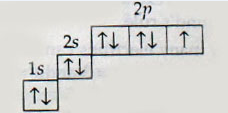
Nitrogen
Oxygen
Fluorine
Neon
Which of the following has the highest mass?
Neutron
Alpha particle
Electron
Deuterium
For a given energy level the number of orbitals is equal to
The relationship between energy of a radiation and its frequency was given by
Planck
Rutherford
Einstein
Joule
The maximum number of electrons in a sub-energy level is equal to
rays are a stream of
He
electrons
positron
neutrons
If value of h is taken as , the de-Broglie wavelength of a particle of mass having velocity is
0.01 m
2 nm
0.1 nm
When the electron is excited from K level to M level we get
- rays
cathode rays
continuous spectra
absorption spectra
Which of the following weighs the least?
1 g of
2 litres of at N.T.P.
molecules of
atoms of carbon
The ion that is isoelectronic with CO is
The possible values of l for an s orbital are
-1, +1
0 to 2
-2 and + 1
0
The atomic number of an element having the valence shell electronic configuration is
35
26
23
34
Davisson and Germer gave an experimental evidence for
wave nature of electron
particle nature of electron
particle nature of light
wave nature of light
