Two substances are in equilibrium in a reversible chemical reaction. If the concentration of each substance is doubled, then the value of the equilibrium constant will be
Same
Doubled
Halved
One fourth of its original value
Correct Answer :
A. Same
Related Questions
Pick out the wrong statement.
The conversion for a gas phase reaction increases with decrease in pressure, if there is an increase in volume accompanying the reaction
With increase in temperature, the equilibrium constant increases for an exothermic reaction
The equilibrium constant of a reaction depends upon temperature only
The conversion for a gas phase reaction increases with increase in pressure, if there is a decrease in volume accompanying the reaction
When a system is in equilibrium for all possible processes, the differential or finite change of entropy is
< 0
> 0
= 0
None of these
A thermodynamic system is taken from state A to B along ACB and is brought back to A along BDA as shown below in the P-V diagram. The net work done during the complete cycle is given by the area covered by
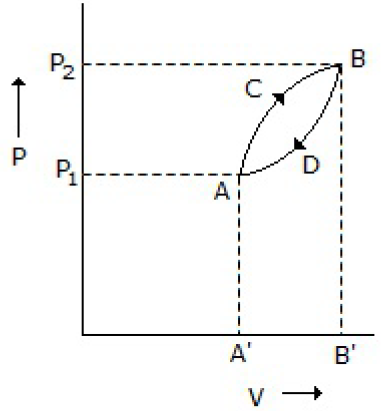
P1ACBP2P1
ACBB1A1A
ACBDA
ADBB1A1A
The internal energy of an ideal gas does not change in a reversible __________ process.
Isothermal
Adiabatic
Isobaric
Isometric
The relation connecting the fugacities of various components in a solution with one another and to composition at constant temperature and pressure is called the __________ equation.
Gibbs-Duhem
Van Laar
Gibbs-Helmholtz
Margules
If the molar heat capacities (Cp or Cv) of the reactants and products of a chemical reaction are identical, then, with the increase in temperature, the heat of reaction will
Increase
Decrease
Remain unaltered
Increase or decrease; depends on the particular reaction
With increase in reduced temperature, the fugacity co-efficient of a gas at constant reduced pressure
Increases
Decreases
Remain same
Decreases linearly
First law of thermodynamics is mathematically stated as
dQ = dE + dW
dQ = dE - dW
dE = dQ + dW
dW = dQ + dE
The change in __________ is equal to the reversible work for compression in steady state flow process under isothermal condition.
Internal energy
Enthalpy
Gibbs free energy
Helmholtz free energy
Pick out the wrong statement.
An ideal liquid or solid solution is defined as one in which each component obeys Raoult's law
If Raoult's law is applied to one component of a binary mixture; Henry's law or Raoult's law is applied to the other component also
Henry's law is rigorously correct in the limit of infinite dilution
None of these
Normal temperature and pressure (N.T.P.) corresponds to
0°C and 760 mm Hg
15°C and 760 mm Hg
20°C and 760 mm Hg
0°C and 1 kgf/cm2
For a stable phase at constant pressure and temperature, the fugacity of each component in a binary system __________ as its mole fraction increases.
Decreases
Increases
Remain same
Decreases linearly
Which of the following is not a unit of the equilibrium constant Kp? (where, Δx = number of moles of products number of moles of reactants)
(atm)Δx, when Δx is negative
(atm)Δx, when Δx is positive
Dimensionless, when Δx = 0
(atm)Δx2, when Δx > 0
Pick out the wrong statement.
The chemical potential of a pure substance depends upon the temperature and pressure
The chemical potential of a component in a system is directly proportional to the escaping tendency of that component
The chemical potential of ith species (μi) in an ideal gas mixture approaches zero as the pressure or mole fraction (xi) tends to be zero at constant temperature
The chemical potential of species 'i' in the mixture (μi) is mathematically represented as,μi = ∂(nG)/∂ni]T,P,nj where, n, ni and nj respectively denote the total number of moles, moles of ith species and all mole numbers except ith species. 'G' is Gibbs molar free energy
Which of the following is affected by the temperature?
Fugacity
Activity co-efficient
Free energy
All (A), (B) & (C)
If the internal energy of an ideal gas decreases by the same amount as the work done by the system, then the
Process must be isobaric
Temperature must decrease
Process must be adiabatic
Both (B) and (C)
The partial molar enthalpy of a component in an ideal binary gas mixture of composition Z, at a temperature T and pressure P, is a function only of
T
T and P
T, P and Z
T and Z
__________ decreases during adiabatic throttling of a perfect gas.
Entropy
Temperature
Enthalpy
Pressure
Critical solution temperature (or the consolute temperature) for partially miscible liquids (e.g., phenol-water) is the minimum temperature at which
A homogeneous solution (say of phenol water) is formed
Mutual solubility of the two liquids shows a decreasing trend
Two liquids are completely separated into two layers
None of these
Gibbs free energy (G) is represented by, G = H - TS, whereas Helmholtz free energy, (A) is given by, A = E - TS. Which of the following is the Gibbs-Helmholtz equation?
[∂(G/T)/∂T] = - (H/T2)
[∂(A/T)/∂T]V = - E/T2
Both (A) and (B)
Neither (A) nor (B)
When a system in equilibrium is subjected to a change in temperature, pressure or concentration, the equilibrium is displaced in a direction which tends to undo the effect of the change. This is called the
Le-Chatelier principle
Kopp's rule
Law of corresponding state
Arrhenius hypothesis
Isentropic process means a constant __________ process.
Enthalpy
Pressure
Entropy
None of these
The partial pressure of each constituent present in an alloy is __________ the total vapor pressure exerted by the alloy.
Less than
Equal to
More than
Either (B) or (C); depends on the type of alloy
Pick out the wrong statement:
The expansion of a gas in vacuum is an irreversible process
An isometric process is a constant pressure process
Entropy change for a reversible adiabatic process is zero
Free energy change for a spontaneous process is negative
Gibbs free energy of mixing at constant pressure and temperature is always
0
∞
+ ve
- ve
In case of the decomposition of hydroiodic acid (2HI H2 + I2), addition of H2 (at equilibrium condition) will
Increase the partial pressure of I2
Decrease the partial pressure of HI
Diminish the degree of dissociation of HI
None of these
Pick out the wrong statement.
A refrigeration cycle violates the second law of thermodynamics
Refrigeration cycle is normally represented by a temperature vs. entropy plot
In a refrigerator, work required decreases as the temperature of the refrigerator and the temperature at which heat is rejected increases
One ton of refrigeration is equivalent to the rate of heat absorption equal to 3.53 kW
The co-efficient of performance (COP) of a refrigerating system, which is its index of performance, is defined as the ratio of useful refrigeration to the net work. The units of __________ and COP are the same.
Kinematic viscosity
Work
Temperature
None of these
Joule-Thomson effect i.e., a throttling process is a constant __________ process.
Entropy
Temperature
Internal energy
Enthalpy
A refrigerator works on the principle of __________ law of thermodynamics.
Zeroth
First
Second
Third
