When cut-off ratio is __________ the efficiency of Diesel cycle approaches to Otto cycle efficiency.
Zero
1/5
4/5
1
Correct Answer :
A. Zero
Related Questions
The heating of gas at constant volume is governed by
Boyle's law
Charles' law
Gay-Lussac law
Avogadro's law
A cycle consisting of __________ and two isothermal processes is known as Stirling cycle.
Two constant pressure
Two constant volume
Two isentropic
One constant pressure, one constant volume
The entropy of water at 0°C is assumed to be
1
0
-1
10
Which of the following is an irreversible cycle?
Carnot
Stirling
Ericsson
None of the above
The general gas equation is (where p = Pressure, v = Volume, m = mass, T = Absolute temperature, and R = Gas constant)
pv = mRT
pv = RTm
pvm = C
pv = (RT)m
Formula based on IS codes is based on
Straight line formula
Eulers formula
Rankines formula
Secant formula
The energy absorbed in a body, when it is strained within the elastic limits, is known as
Strain energy
Resilience
Proof resilience
Modulus of resilience
Mond gas is obtained by
Partial combustion of coal, coke, anthracite coal or charcoal in a mixed air steam blast
Carbonisation of bituminous coal
Passing steam over incandescent coke
Passing air and a large amount of steam over waste coal at about 650°C
Which of the following statement is correct?
The heat and work are boundary phenomena
The heat and work represent the energy crossing the boundary of the system
The heat and work are path functions
All of the above
The compression ratio for petrol engines is
3 to 6
5 to 8
15 to 20
20 to 30
Stirling cycle consists of
Two constant volume and two isentropic processes
Two constant volume and two isothermal processes
Two constant pressure and two isothermal processes
One constant volume, one constant pressure and two isentropic processes
Which of the following gas has the highest calorific value?
Coal gas
Producer gas
Mond gas
Blast furnace gas
The most probable velocity of the gas molecules is given by
√(KT/m)
√(2KT/m)
√(3KT/m)
√(5KT/m)
The __________ states that change of internal energy of a perfect gas is directly proportional to the change of temperature.
Boyle's law
Charle's law
Gay-Lussac law
Joule's law
The calorific value of gaseous fuel is expressed in
kJ
kJ/kg
kJ/m2
kJ/m3
A cylindrical section having no joint is known as
Joint less section
Homogeneous section
Perfect section
Seamless section
When a thin cylindrical shell is subjected to an internal pressure, the volumetric strain is (where ε₁ = Hoop strain, and ε₂ = Longitudinal strain)
2ε₁ - ε₂
2ε₁ + ε₂
2ε₂ - ε₁
2ε₂ + ε₁
For same compression ratio and for same heat added
Otto cycle is more efficient than Diesel cycle
Diesel cycle is more efficient than Otto cycle
Efficiency depends on other factors
Both Otto and Diesel cycles are equally efficient
Reversed Joule cycle is known as
Carnot cycle
Bell-Coleman cycle
Rankine cycle
Stirling cycle
All perfect gases change in volume by 1/273th of its original volume at 0°C for every 1°C change in temperature, when the pressure remains constant. This statement is called
Boyle's law
Charles' law
Gay-Lussac law
Joule's law
The value of Poisson's ratio for steel is between
0.01 to 0.1
0.23 to 0.27
0.25 to 0.33
0.4 to 0.6
The variables which control the physical properties of a perfect gas are
Pressure exerted by the gas
Volume occupied by the gas
Temperature of the gas
All of these
The main cause for the irreversibility is
Mechanical and fluid friction
Unrestricted expansion
Heat transfer with a finite temperature difference
All of the above
A molecule consisting of one atom is known as
Mono-atomic
Di-atomic
Tri-atomic
Poly-atomic
The isothermal and adiabatic processes are regarded as
Reversible process
Irreversible process
Reversible or irreversible process
None of these
A key is subjected to side pressure as well at shearing forces. These pressures are called
Bearing stresses
Fatigue stresses
Crushing stresses
Resultant stresses
The air standard efficiency of an Otto cycle is given by (where r = Compression ratio, and γ = Ratio of specific heats)
1 - rγ - 1
1 + rγ - 1
1 - (1/ rγ - 1)
1 + (1/ rγ - 1)
Which of the following is correct?
Absolute pressure = Gauge pressure + Atmospheric pressure
Gauge pressure = Absolute pressure + Atmospheric pressure
Atmospheric pressure = Absolute pressure + Gauge pressure
Absolute pressure = Gauge pressure - Atmospheric pressure
In the below figure, the stress corresponding to point D is
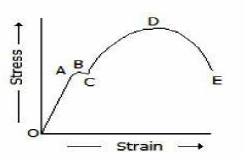
Yield point stress
Breaking stress
Ultimate stress
Elastic limit
The smallest quantity of a substance, which can exist by itself in a chemically recognizable form is known as
Element
Compound
Atom
Molecule
