When liquid and vapour phase of multi-component system are in equilibrium (at a given temperature and pressure), then chemical potential of each component is
Same in both the phases
Zero in both the phases
More in vapour phase
More in liquid phase
Correct Answer :
A. Same in both the phases
Related Questions
Chemical potential (an intensive property) of a substance is a force that drives the chemical system to equilibrium and is equal to its partial molar properties. The ratio of chemical potential to free energy of a pure substance at constant temperature and pressure is
0
1
∞
None of these
Enthalpy of a gas depends upon its
Temperature
Mass
Volume
Pressure
Those solutions in which there is no volume change upon mixing the components in the liquid state and which, when diluted do not undergo any heat change (i.e. heat of dilution is zero), are called __________ solutions.
Ideal
Real
Isotonic
None of these
Which is a state function?
Specific volume
Work
Pressure
Temperature
When a gas is subjected to adiabatic expansion, it gets cooled due to
Decrease in velocity
Decrease in temperature
Decrease in kinetic energy
Energy spent in doing work
In an irreversible process
Tds = dE - dW = 0
dE - dW - Tds = 0
Tds - dE + dW < 0
Tds - dT + dW < 0
PVγ = Constant (where, γ = Cp/Cv) is valid for a/an __________ process.
Isothermal
Isentropic
Isobaric
Adiabatic
For multi-component multiple phases to be in equilibrium at the same pressure and temperature, the __________ of each component must be same in all phases.
Chemical potential
Fugacity
Both (A) and (B)
Neither (A) nor (B)
An isentropic process is carried out at constant
Volume
Pressure
Temperature
All (A), (B) and (C)
Pick out the wrong statement.
A refrigeration cycle violates the second law of thermodynamics
Refrigeration cycle is normally represented by a temperature vs. entropy plot
In a refrigerator, work required decreases as the temperature of the refrigerator and the temperature at which heat is rejected increases
One ton of refrigeration is equivalent to the rate of heat absorption equal to 3.53 kW
When a gas is expanded from high pressure region to low pressure region; temperature change occurs. This phenomenon is related to the
Gibbs-Duhem equation
Gibbs-Helmholtz equation
Third law of thermodynamics
Joule-Thomson effect
The change in Gibbs free energy for vaporisation of a pure substance is
Positive
Negative
Zero
May be positive or negative
The melting point of paraffin wax (which contracts on solidification) __________ with pressure rise.
Increases
Decreases
Remains unchanged
Decreases linearly
In case of vapour compression refrigeration system, elevating the evaporator temperature (keeping the condenser temperature constant) results in
Enhanced COP
Decreased COP
No change in the value of COP
Increased or decreased COP; depending upon the type of refrigerant
The equation, Cp - Cv = R, is true for __________ gas.
No
Any real
Only ideal
Both (B) and (C)
Which of the following is not a unit of the equilibrium constant Kp? (where, Δx = number of moles of products number of moles of reactants)
(atm)Δx, when Δx is negative
(atm)Δx, when Δx is positive
Dimensionless, when Δx = 0
(atm)Δx2, when Δx > 0
Which of the following diagrams does not represent an Otto cycle?
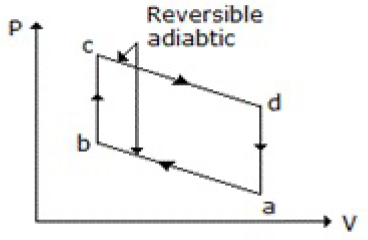
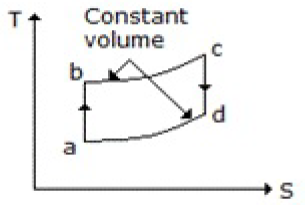
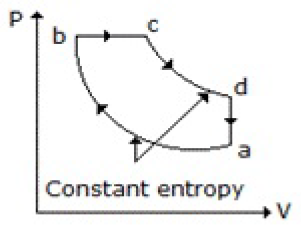
None of these
High __________ is an undesirable property for a good refrigerant.
Specific heat
Latent heat of vaporisation
Viscosity
Specific vapor volume
Sound waves propagation in air exemplifies an __________ process.
Adiabatic
Isothermal
Isometric
None of these
Solid and liquid phases of a substance are in equilibrium at the
Critical temperature
Melting point
Freezing point
Both (B) and (C)
When a system in equilibrium is subjected to a change in temperature, pressure or concentration, the equilibrium is displaced in a direction which tends to undo the effect of the change. This is called the
Le-Chatelier principle
Kopp's rule
Law of corresponding state
Arrhenius hypothesis
Co-efficient of Performance (COP) of a refrigerator is the ratio of the
Work required to refrigeration obtained
Refrigeration obtained to the work required
Lower to higher temperature
Higher to lower temperature
Steam undergoes isentropic expansion in a turbine from 5000 kPa and 400°C (entropy = 6.65 kJ/kg K) to 150 kPa) (entropy of saturated liquid = 1.4336 kJ/kg. K, entropy of saturated vapour = 7.2234 kJ/kg. K) The exit condition of steam is
Superheated vapour
Partially condensed vapour with quality of 0.9
Saturated vapour
Partially condensed vapour with quality of 0.1
At absolute zero temperature, the __________ of the gas is zero.
Pressure
Volume
Mass
None of these
__________ functions are exemplified by heat and work.
Path
Point
State
None of these
Molar heat capacity of water in equilibrium with ice at constant pressure is __________ Kcal/kg mole. °K
0
∞
50
100
Entropy change of mixing two liquid substances depends upon the
Molar concentration
Quantity (i.e. number of moles)
Both (A) and (B)
Neither (A) nor (B)
Mollier chart is a __________ plot.
Pressure vs. enthalpy
Pressure vs. volume
Enthalpy vs. entropy
Temperature vs. entropy
A refrigerator may be termed as a
Heat pump
Heat engine
Carnot engine
None of these
Entropy of an ideal gas depends upon its
Pressure
Temperature
Both (A) & (B)
Neither (A) nor (B)
