Which of the following is a proper sequence?
Proportional limit, elastic limit, yielding, failure
Elastic limit, proportional limit, yielding, failure
Yielding, proportional limit, elastic limit, failure
None of the above
Correct Answer :
A. Proportional limit, elastic limit, yielding, failure
Related Questions
One kg of carbon requires 4/3 kg of oxygen and produces __________ kg of carbon monoxide gas.
8/3
11/3
11/7
7/3
A process, in which the gas is heated or expanded in such a way that the product of its pressure and volume remains constant, is called
Isothermal process
Hyperbolic process
Adiabatic process
Polytropic process
An isothermal process is governed by
Boyle's law
Charles' law
Gay-Lussac law
Avogadro's law
The bending equation is
M/I = σ/y = E/R
T/J = τ/R = Cθ/l
M/R = T/J = Cθ/l
T/l= τ/J = R/Cθ
When a body is subjected to a direct tensile stress (σx) in one plane accompanied by a simple shear stress (τxy), the minimum normal stress is
(σx/2) + (1/2) × √(σx² + 4 τ²xy)
(σx/2) - (1/2) × √(σx² + 4 τ²xy)
(σx/2) + (1/2) × √(σx² - 4 τ²xy)
(1/2) × √(σx² + 4 τ²xy)
In a free expansion process,
W1 - 2 = 0
Q1 - 2 = 0
dU = 0
All of these
The general gas equation is (where p = Pressure, v = Volume, m = mass, T = Absolute temperature, and R = Gas constant)
pv = mRT
pv = RTm
pvm = C
pv = (RT)m
A cube subjected to three mutually perpendicular stress of equal intensity p expenses a volumetric strain
3p/E × (2/m - 1)
3p/E × (2 - m)
3p/E × (1 - 2/m)
E/3p × (2/m - 1)
The efficiency of the Carnot cycle may be increased by
Increasing the highest temperature
Decreasing the highest temperature
Increasing the lowest temperature
Keeping the lowest temperature constant
One kg of hydrogen requires 8 kg of oxygen and produces
1 kg of water
7 kg of water
8 kg of water
9 kg of water
The mass of carbon per kg of flue gas is given by
(11/3) CO2 + (3/7) CO
(3/7) CO2 + (11/3) CO
(7/3) CO2 + (3/11) CO
(3/11) CO2 + (7/3) CO
The heating of gas at constant volume is governed by
Boyle's law
Charles' law
Gay-Lussac law
Avogadro's law
The total elongation produced in a bar of uniform section hanging vertically downwards due to its own weight is equal to that produced by a weight
Of same magnitude as that of bar and applied at the lower end
Half the weight of bar applied at lower end
Half of the square of weight of bar applied at lower end
One fourth of weight of bar applied at lower end
In a reversible adiabatic process, the ratio of T1/T2 is equal to
(p2/p1)γ - 1/ γ
(p1/p2)γ - 1/ γ
(v2/v1)γ - 1/ γ
(v1/v2)γ - 1/ γ
The given figure shows the Mohr's circle of stress for two unequal and like principal stresses (σx and σy) acting at a body across two mutually perpendicular planes. The normal stress on an oblique section making an angle θ with the minor principle plane is given by
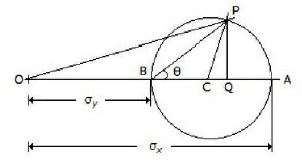
OC
OP
OQ
PQ
The deformation of a bar under its own weight is _________ the deformation, if the same body is subjected to a direct load equal to weight of the body.
Equal to
Half
Double
Quadruple
Otto cycle is also known as
Constant pressure cycle
Constant volume cycle
Constant temperature cycle
Constant temperature and pressure cycle
The most probable velocity of the gas molecules is given by
√(KT/m)
√(2KT/m)
√(3KT/m)
√(5KT/m)
The entropy of water at 0°C is assumed to be
1
0
-1
10
Coke is produced
When coal is first dried and then crushed to a fine powder by pulverising machine
From the finely ground coal by moulding under pressure with or without a binding material
When coal is strongly heated continuously for 42 to 48 hours in the absence of air in a closed vessel
By heating wood with a limited supply of air to a temperature not less than 280°C
One molecule of oxygen consists of __________ atoms of oxygen.
2
4
8
16
The efficiency of Joule cycle is
Greater than Carnot cycle
Less than Carnot cycle
Equal to Carnot cycle
None of these
During which of the following process does heat rejection takes place in Carnot cycle?
Isothermal expansion
Isentropic expansion
Isothermal compression
Isentropic compression
Workdone in a free expansion process is
Zero
Minimum
Maximum
Positive
Which of the following cycles has maximum efficiency?
Rankine
Stirling
Carnot
Brayton
A coil is cut into two halves, the stiffness of cut coil will be
Double
Half
Same
None of these
Which of the following is the correct statement?
All the reversible engines have the same efficiency.
All the reversible and irreversible engines have the same efficiency.
Irreversible engines have maximum efficiency.
All engines are designed as reversible in order to obtain maximum efficiency.
The rivets are used for __________ fastenings.
Permanent
Temporary
Semi-permanent
None of these
The ratio of molar specific heats for mono-atomic gas is
1
1.4
1.67
1.87
According to Kelvin-Planck's statement of second law of thermodynamics,
It is impossible to construct an engine working on a cyclic process, whose sole purpose is to convert heat energy into work
It is possible to construct an engine working on a cyclic process, whose sole purpose is to convert heat energy into work
It is impossible to construct a device which operates in a cyclic process and produces no effect other than the transfer of heat from a cold body to a hot body
None of the above
