In the case of atomic spectrum of hydrogen which series of lines lie in the visible region?
Balmer
Paschen
Pfund
None of these
Correct Answer :
B. Paschen
Related Questions
Which elements is represented by the following electronic configuration?
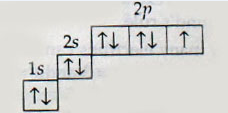
Nitrogen
Oxygen
Fluorine
Neon
The fundamental particle that has least mass is
meson
alpha-particle
electron
neutron
A dipositive ion has in the K shell, 8 e~ s in the L shell and in the M shell. Atomic number of Z is
19
20
16
15
Which of the following has the highest mass?
Neutron
Alpha particle
Electron
Deuterium
Which of the following atoms has a non-spherical outermost orbital.
H
Li
Be
B
Isotones have same
mass number
nuclear mass
no. of neutrons
no. of electrons
The wavelength of an electron
is equal to that of light
remains constant with velocity
decreases with an increasing velocity
increases with an decreasing velocity
Davisson and Germer gave an experimental evidence for
wave nature of electron
particle nature of electron
particle nature of light
wave nature of light
The atomic number of an element having the valence shell electronic configuration is
35
26
23
34
In Lyman series an electron jumps from higher energy level to
K energy level
M energy level
N energy level
L energy level
The orbitals with maximum number of possible orientation is:
s
d
f
p
In the nucleus of there are
40 protons and 20 electrons
20 protons and 40 electrons
20 protons and 40 neutrons
20 protons and 20 neutrons
No two electrons in an atom can have the same values of all four quantum numbers according to
Hund's rules
Flemming rule
Pauli's exclusion principle
Bohr theory
The corect set of quantum numbers for the impaired electron of fluorine atom is
| n | l | m |
|---|---|---|
| 2 | 1 | 0 |
| n | l | m |
|---|---|---|
| 2 | 1 | 1 |
| n | l | m |
|---|---|---|
| 3 | 1 | 1 |
| n | l | m |
|---|---|---|
| 3 | 0 | 0 |
Among the following ions, which are has the highest paramagnetism?
Charge on an electron was shown by
J. J. Thomson
Kirchoff
Ohm
M.Planck
Bohr's concept of the orbit in an atom was contradicted by
de Broglie relationship
Uncertainty principle
Planck's hypothesis
Hund's rule
When the electron is excited from K level to M level we get
- rays
cathode rays
continuous spectra
absorption spectra
The exchange of particles considered responsible for keeping the nucleons together are
meson
electron
positron
neutron
The ion that is isoelectronic with CO is
Cathode rays contain a stream of
The number of unpaired electrons in the ground state of chromium is
1
6
7
2
Charge on fundamental particle neutrino is
0
+1
-1
None of these
The size of nucleus is
The relationship between energy of a radiation and its frequency was given by
Planck
Rutherford
Einstein
Joule
The velocity of a photon is
dependent on its wavelength
dependent on its source
equal to cube of its amplitude
independent of its wavelength
The angular momentum of the moving electron is equal to
The maximum number of electrons in a sub-energy level is equal to
Mass of positron is the same to that of
proton
meson
electron
neutron
The maximum number of electrons that can be accommodated by an atom in g-sub-energy level are
20
25
18
12
