Direction.
Directions: Are based on the graph given below:
Solubility-Temperature relationships for various salts.
(The Y-axis denotes Solubility (kg/litre of water))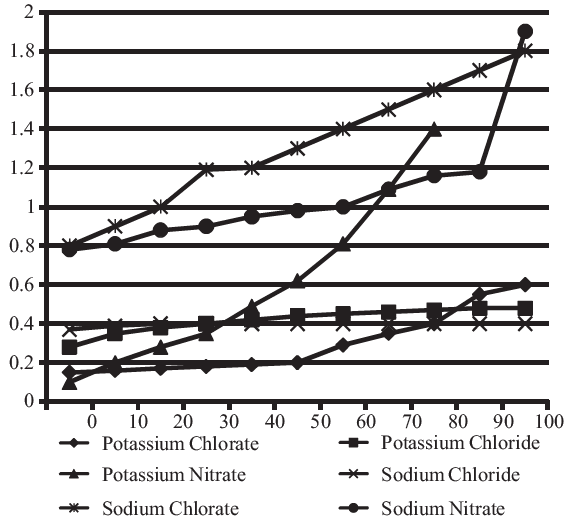
If 1 mole of Potassium Chloride weighs 0.07456 kg, approximately. How many moles of Potassium Chloride can be dissolved in 100 litres of water at 36°C?
700
650
480
540
Correct Answer :
D. 540
Solubility of potassium chloride at 36°C = 0.4 kg./lt. so, in 100lir solution the amount of Potassium chloride dissolved in = 40 kg. Number of moles = 40 / 0.075 = 533 approx. 540 moles can be dissolved in 100 lt. of water at 36°C.
Related Questions
Suppose that each widget sells for Rs. 150. What is the profit earned by ABC Ltd. in July? (Profit is defined as the excess of sales revenue over total cost.)
2400
1600
400
0
In 2004, the prices of plywood, sawn timber and logs went up by 5%, 1% and 10% respectively and the total sales were made up of 40% plywood, 30% sawn timber and 30% logs. The average realization per cubic metre in 2004 was closest to
Rs. 15,500
Rs. 16,135.5
Rs. 14,500
Rs. 18,500
What is the gross profit in 1993?
Rs. 27
Rs. 30
Rs. 35
None of these
The number of men who joined the club in 2010 was what % of the total number of people who joined the club in the same year?
0.63
0.75
0.27
0.11
In which year women and men ratio is greatest?
2011
2012
2013
None of these
The number of males passing out from colleges A and B together is what percent of the number of females passing out from colleges C and D together?
50
45
40
35
ABC Ltd. is considering increasing the production level. What is the approximate marginal cost of increasing production from its July level of 40 units?
110
130
150
160
If there were 30 % old men (approx.) in the club in 2013 then what is the percentage of new young men to the young men in the group in 2014, considering there were no old man join the club?
74.52
57.36
39.24
18.07
If the profit earned by the company in the year 2008 was 90,000, what was the income of the company in that year?
2,90,000
2,00,000
1,50,000
Cannot be determined
In which year is the profit per rupee of equity the highest?
1991
1992
1993
1990 1991
In which of the following years was the production of motorbikes exactly 40% of the total production of automobiles in that years?
1997
2000
1999
1996
What was the ratio between the number girls enrolled in the school-C in the year 2007 and the total number of girls enrolled in school-A and school-B together in the same year?
11 : 3
3 : 11
4 : 11
4 : 7
In how many months out of the given 7 months expenditure in 1998 is more than that in 1997 but less than that in 1999.
2
3
4
5
Which of the salts has greater change in solubility in kg / litre of water between 15°C and 25°C?
Potassium Chlorate
Potassium Nitrate
Sodium Chlorate
Sodium Nitrate
If the profit was Rs. 600 in 1993, then what was the profit in 1990?
Rs. 441
Rs. 395
Rs. 480
Rs. 545
What is the average number of students (males and females) passed out from all the colleges together?
38000
48000
42000
None of these
Assume that the unit price is Rs. 150 and profit is defined as the excess of sales revenue over total costs. What is the monthly production level of ABC Ltd. at which the profit is highest?
30
50
60
40
In which value score, there exists convergence between personal profile and average female profile?
Theoretical
Social
Aesthetic
None of the above
The expenditure in April 1999 was . . . . . . . . higher than that of corresponding period in 1998.
1.5%
2%
2.5%
0.94%
In which of the following years, there was the maximum net growth in car sales as compared to its earlier years?
1994
1992
1993
1995
The rate of growth during the third month was the lowest for
Geeta
Seeta
Ram
Shyam
The number of educated and illiterate members (not highly educated) who joined the club in 2013 as a percentage of total number of men who joined the club in the same year could not be more than
11.43
15.65
9.63
17.34
In 2003, the total sales of the company measured in cubic metres was made up of 40% plywood, 30% sawn timber and 30% logs. The average realization per cubic metre in 2003 was closest to
Rs. 16,500
Rs. 13,500
Rs. 15,425
Rs. 18,000
With profitability as defined in question 137, it can be concluded that
Profitability is non-decreasing during the five years from 1994-95 to 1998-99.
Profitability is non-increasing during the five years from 1994-95 to 1998-99.
Profitability remained constant during the five years from 1994-95 to 1998-99.
None of the above
In which month is the total increase in the cost highest as compared to two months ago?
March
September
July
May
What is the average per cent profit earned by the company over the years?
55
51
62
59
The number of females passed out from college C is approximately what percent of the total number of females passed out from all the colleges together?
28
30
36
25
If the trend observed between 1999 and 2000 continues in the next year, what will be number of students passing in the examination in 2001?
245
237
263
300
The number of students keeps on increasing by 50 every years. In 1998, there were 250 students. For which of the following years is the performance best in the school?
1998
2000
1999
Cannot be determined
If sales were Rs. 1200 crore in 1990, then what were the total sales in the period 1990-1995?
Rs. 8628 crore
Rs. 9828 crore
Rs. 9156 crore
Rs. 8136 crore
