Internal energy is equal to the heat absorbed in case of a/an __________ process.
Constant volume
Polytropic
Adiabatic
Constant pressure
Correct Answer :
A. Constant volume
Related Questions
The theoretical minimum work required to separate one mole of a liquid mixture at 1 atm, containing 50 mole % each of n- heptane and noctane into pure compounds each at 1 atm is
-2 RT ln 0.5
-RT ln 0.5
0.5 RT
2 RT
A liquid under pressure greater than its vapour pressure for the temperature involved is called a __________ liquid.
Sub-cooled
Saturated
Non-solidifiable
None of these
As the time is passing, entropy of the universe
Is increasing
Is decreasing
Remain constant
Data insufficient, can't be predicted
In a homogeneous solution, the activity coefficient of a component depends upon the
Pressure
Composition
Temperature
All (A), (B) and (C)
If the internal energy of an ideal gas decreases by the same amount as the work done by the system, then the
Process must be isobaric
Temperature must decrease
Process must be adiabatic
Both (B) and (C)
In case of a close thermodynamic system, there is __________ across the boundaries.
No heat and mass transfer
No mass transfer but heat transfer
Mass and energy transfer
None of these
When a gas in a vessel expands, its internal energy decreases. The process involved is
Reversible
Irreversible
Isothermal
Adiabatic
Number of phases in a colloidal system is:
1
2
3
4
A cylinder contains 640 gm of liquid oxygen. The volume occupied (in litres) by the oxygen, when it is released and brought to standard conditions (0°C, 760 mm Hg) will be __________ litres.
448
224
22.4
Data insufficient; can't be computed
Maxwell's relation corresponding to the identity, dH = dS = Vdp + Σμi dni is
(∂T/∂V)S, ni = -(∂P/∂S)V, ni
(∂S/∂P)T, ni = (∂V/∂T)P, ni
(∂S/∂V)T, ni = (∂P/∂T)V, ni
(∂T/∂P)S, ni = (∂V/∂S)P, ni
PVγ = Constant (where, γ = Cp/Cv) is valid for a/an __________ process.
Isothermal
Isentropic
Isobaric
Adiabatic
Pick out the correct statement.
The available energy in an isolated system for all irreversible (real) processes decreases
The efficiency of a Carnot engine increases, if the sink temperature is decreased
The reversible work for compression in non-flow process under isothermal condition is the change in Helmholtz free energy
All (A), (B) and (C)
Which of the following diagrams does not represent an Otto cycle?
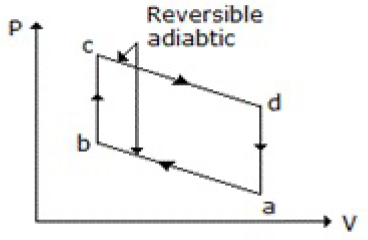
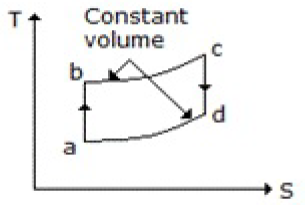
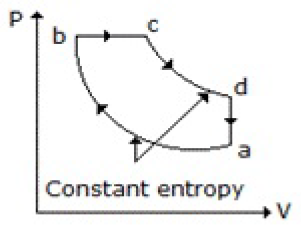
None of these
Two substances are in equilibrium in a reversible chemical reaction. If the concentration of each substance is doubled, then the value of the equilibrium constant will be
Same
Doubled
Halved
One fourth of its original value
Gibbs free energy of mixing at constant pressure and temperature is always
0
∞
+ ve
- ve
The equation Tds = dE - PdV applies to
Single phase fluid of varying composition
Single phase fluid of constant composition
Open as well as closed systems
Both (B) and (C)
Dryness fraction of wet steam is defined as the ratio of mass of vapour in the mixture to the mass of mixture __________ calorimeter is not used for measuring the dryness fraction of steam.
Bomb
Separating
Bucket
Throttling
The partial pressure of each constituent present in an alloy is __________ the total vapor pressure exerted by the alloy.
Less than
Equal to
More than
Either (B) or (C); depends on the type of alloy
1m3 of an ideal gas at 500 K and 1000 kPa expands reversibly to 5 times its initial volume in an insulated container. If the specific heat capacity (at constant pressure) of the gas is 21 J/mole . K, the final temperature will be
35 K
174 K
274 K
154 K
At __________ point, all the three phases (i.e. solid, liquid and gas) co-exist.
Eutectic
Triple
Plait
Critical
Heat requirement for decomposition of a compound into its elements is __________ that is evolved during the formation of that compound from its elements.
The same
Less than
Greater than
Different than
A cyclic engine exchanges heat with two reservoirs maintained at 100 and 300°C respectively. The maximum work (in J) that can be obtained from 1000 J of heat extracted from the hot reservoir is
349
651
667
1000
The freezing point of a liquid decreases when the pressure is increased, if the liquid __________ while freezing.
Contracts
Expands
Does not change in volume
Either (A), (B) or (C)
Equation which relates pressure, volume and temperature of a gas is called the
Equation of state
Gibbs Duhem equation
Ideal gas equation
None of these
What is the ratio of adiabatic compressibility to isothermal compressibility?
1
< 1
> 1
>> 1
When a system is in equilibrium for all possible processes, the differential or finite change of entropy is
< 0
> 0
= 0
None of these
Pick out the wrong statement.
An ideal liquid or solid solution is defined as one in which each component obeys Raoult's law
If Raoult's law is applied to one component of a binary mixture; Henry's law or Raoult's law is applied to the other component also
Henry's law is rigorously correct in the limit of infinite dilution
None of these
Normal temperature and pressure (N.T.P.) corresponds to
0°C and 760 mm Hg
15°C and 760 mm Hg
20°C and 760 mm Hg
0°C and 1 kgf/cm2
Entropy of a substance remains constant during a/an __________ change.
Reversible isothermal
Irreversible isothermal
Reversible adiabatic
None of these
The melting point of paraffin wax (which contracts on solidification) __________ with pressure rise.
Increases
Decreases
Remains unchanged
Decreases linearly
