The isothermal and adiabatic processes are regarded as
Reversible process
Irreversible process
Reversible or irreversible process
None of these
Correct Answer :
A. Reversible process
Related Questions
During which of the following process does heat rejection takes place in Carnot cycle?
Isothermal expansion
Isentropic expansion
Isothermal compression
Isentropic compression
A fletched beam is used to
Change the shape of the beam
Effect the saving in material
Equalise the strength in tension and compression
Increase the cross-section of the beam
The property of a material by virtue of which it can be beaten or rolled into plates is called
Malleability
Ductility
Plasticity
Elasticity
The efficiency of a gas turbine is given by
(Net work output)/(Workdone by the turbine)
(Net work output)/(Heat supplied)
(Actual temperature drop)/(Isentropic temperature drop)
(Isentropic increase in temperature)/(Actual increase in temperature)
When a bar is cooled to - 5°C, it will develop
No stress
Shear stress
Tensile stress
Compressive stress
The torsional rigidity of a shaft is expressed by the
Maximum torque it can transmit
Number of cycles it undergoes before failure
Elastic limit up to which it resists torsion, shear and bending stresses
Torque required to produce a twist of one radian per unit length of shaft
In compression test, the fracture in cast iron specimen would occur along
The axis of load
An oblique plane
At right angles to the axis of specimen
Would not occur
The mass of flue gas per kg of fuel is the ratio of the
Mass of oxygen in 1 kg of flue gas to the mass of oxygen in 1 kg of fuel
Mass of oxygen in 1 kg of fuel to the mass of oxygen in 1 kg of flue gas
Mass of carbon in 1 kg of flue gas to the mass of carbon in 1 kg of fuel
Mass of carbon in 1 kg of fuel to the mass of carbon in 1 kg of flue gas
When two plates are butt together and riveted with cover plates with two rows of rivets, the joint is known as
Lap joint
Butt joint
Single riveted single cover butt joint
Double riveted double cover butt joint
In the below figure, curve D represents_________.
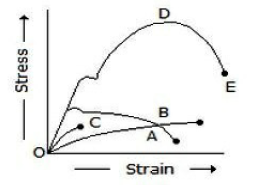
Mild steel
Cast iron
Concrete
Bone of these
One kg of carbon requires 4/3 kg of oxygen and produces __________ kg of carbon monoxide gas.
8/3
11/3
11/7
7/3
The ratio of molar specific heats for mono-atomic gas is
1
1.4
1.67
1.87
The mass of excess air supplied is equal to
(23/100) × Mass of excess carbon
(23/100) × Mass of excess oxygen
(100/23) × Mass of excess carbon
(100/23) × Mass of excess oxygen
Which of the following process can be made reversible with the help of a regenerator?
Constant pressure process
Constant volume process
Constant pvn process
All of these
The energy stored in a body when strained within elastic limit is known as
Resilience
Proof resilience
Strain energy
Impact energy
When a system changes its state from one equilibrium state to another equilibrium state, then the path of successive states through which the system has passed, is known as
Thermodynamic law
Thermodynamic process
Thermodynamic cycle
None of these
The strain energy stored in a body due to suddenly applied load compared to when it is applied gradually is
Same
Twice
Four times
Eight times
The variables which control the physical properties of a perfect gas are
Pressure exerted by the gas
Volume occupied by the gas
Temperature of the gas
All of these
The sum of internal energy (U) and the product of pressure and volume (p.v) is known as
Workdone
Entropy
Enthalpy
None of these
When the gas is heated at constant volume, the heat supplied
Increases the internal energy of the gas and increases the temperature of the gas
Does some external work during expansion
Both (A) and (B)
None of these
The efficiency of the dual combustion cycle for the same compression ratio is __________ Diesel cycle.
Greater than
Less than
Equal to
None of these
For which material the Poisson's ratio is more than unity
Steel
Copper
Aluminium
None of the above
In open cycle gas turbine plants
The indirect heat exchanger and cooler is avoided
Direct combustion system is used
A condenser is used
All of the above
The absolute zero pressure can be attained at a temperature of
0°C
273°C
273 K
None of these
The strain energy stored in a solid circular shaft subjected to shear stress (τ), is: (Where G = Modulus of rigidity for the shaft material)
τ²/ 2G × Volume of shaft
τ/ 2G × Volume of shaft
τ²/ 4G × Volume of shaft
τ/ 4G × Volume of shaft
The shear force diagram of a cantilever beam of length l and carrying a uniformly distributed load of w per unit length will be
A right angled triangle
An isosceles triangle
An equilateral triangle
A rectangle
The distillation carried out in such a way that the liquid with the lowest boiling point is first evaporated and recondensed, then the liquid with the next higher boiling point is then evaporated and recondensed, and so on until all the available liquid fuels are separately recovered in the sequence of their boiling points. Such a process is called
Cracking
Carbonisation
Fractional distillation
Full distillation
An open system is one in which
Heat and work crosses the boundary of the system, but the mass of the working substance does not crosses the boundary of the system
Mass of the working substance crosses the boundary of the system but the heat and work does not crosses the boundary of the system
Both the heat and work as well as mass of the working substance crosses the boundary of the system
Neither the heat and work nor the mass of the working substance crosses the boundary of the system
Second law of thermodynamics defines
Heat
Work
Internal energy
Entropy
The atomic mass of sulphur is
12
14
16
32
