Direction.
Directions: Are based on the graph given below:
Solubility-Temperature relationships for various salts.
(The Y-axis denotes Solubility (kg/litre of water))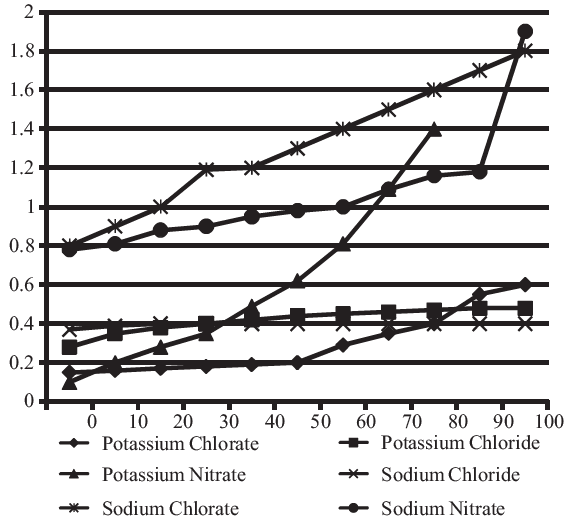
Approximately, how many kg of Potassium Nitrate can be dissolved in 10 litres of water at 30°C?
0.04
0.4
4
0.35
Correct Answer :
C. 4
At 30°C, solubility of potassium nitrate is 0.38 kg./lt. Hence in 10 lt. 3.8 kg., Approx. = 4 kg. of potassium nitrate can be dissolved.
Related Questions
If the expenditure of the company in the year 2006 was 75,000, what was the ratio of income to expenditure of the company in that year?
3 : 2
5 : 4
4 : 3
Cannt be determined
In which year was the increase in spending on CSR, vis-a-vis the previous year, the maximum?
2006
2007
2008
2009
Which month has the highest profit per employee?
September
July
January
March
In which year is the profit per rupee of equity the highest?
1991
1992
1993
1990 1991
If sales were Rs. 1200 crore in 1990, then what were the total sales in the period 1990-1995?
Rs. 8628 crore
Rs. 9828 crore
Rs. 9156 crore
Rs. 8136 crore
Approximately, how many kg of Potassium Nitrate can be dissolved in 10 litres of water at 30°C?
0.04
0.4
4
0.35
What was the difference between the average sales index and the average cost index?
7.3
7.7
7.5
7.9
Mr.X, a funds manager with an investment company invested 25% of his funds in each of the four commodities at the beginning of the period. He sold the commodities at the end of the period. His investments in the commodities resulted in:
17% profit
5.5% loss
no profit, no loss
3% profit
The number of males passing out from colleges A and B together is what percent of the number of females passing out from colleges C and D together?
50
45
40
35
In the given personal profile, which is the value with the lowest score?
Theoretical
Religious
Social
Aesthetic
In which year was the total number of girls enrolled in all the three schools together second highest?
2005
2006
2007
2008
Which share showed the greatest percentage increase in market value in any month during the entire period?
A
B
C
D
For monthly production level in the range of 0 to 30 units,
AC is always higher than MC.
AC is always lower than MC.
AC is lower than MC up to a certain level and then is higher than MC.
None of the above is true.
Assuming that no employees left the job, how many more people did the company take on in the given period?
4,600
5,000
5,800
6,400
In which value score, there exists a no difference state between the personal profile and average male profile?
Economic
Social
Aesthetic
None of the above.
In which year, were the gross sales proceeds the highest?
1992
1993
1994
1995
The total amount of profit made by the departmental store increased by approximately what percent from 1997 to 2000 ?
40%
50%
90%
120%
If the profit was Rs. 600 in 1993, then what was the profit in 1990?
Rs. 441
Rs. 395
Rs. 480
Rs. 545
If 1 mole of Potassium Chloride weighs 0.07456 kg, approximately. How many moles of Potassium Chloride can be dissolved in 100 litres of water at 36°C?
700
650
480
540
Which of the following salts has greatest solubility?
Potassium Chlorate at 80°C.
Potassium Chloride at 35°C.
Potassium Nitrate at 39°C.
Sodium Chloride at 85°C.
If the profit earned by the company in the year 2008 was 90,000, what was the income of the company in that year?
2,90,000
2,00,000
1,50,000
Cannot be determined
If the amount invested by the two companies in 2005 was equal, what was the ratio of the total income of the company A to that of B in 2005?
31 : 33
33 : 31
34 : 31
14 : 11
Who grew at the fastest rate in the first two months of life?
Geeta
Seeta
Ram
Shyam
In which month was the greatest absolute change in market value for any share recorded?
March
April
May
June
In which value score, there exists convergence between personal profile and average female profile?
Theoretical
Social
Aesthetic
None of the above
In which year, the maximum profit was generated vis-�-vis in?
1991
1992
1993
1994
The highest percentage of growth in sales, relative to the previous year, occurred in
1995-96
1996-97
1997-98
1998-99
In which of the following quarters, did the departmental store make the least amount of profits?
Third quarter of 2000
Second quarter of 1999
First quarter of 1999
Third quarter of 1998
What total expenditure has been made during the year 1997 and 1998 in the period covered in the graph?
42,87,000
2,70,000
48,27,000
42,78,000
The performance for which of the following houses is the best?
Pearl
Ruby
Topaz
Sapphire
