Entropy of an ideal gas depends upon its
Pressure
Temperature
Both (A) & (B)
Neither (A) nor (B)
Correct Answer :
C. Both (A) & (B)
Related Questions
Fundamental principle of refrigeration is based on the __________ law of thermodynamics.
Zeroth
First
Second
Third
The number of degree of freedom for an Azeotropic mixture of ethanol and water in vapourliquid equilibrium, is
3
1
2
0
With increase in pressure (above atmospheric pressure), the Cp of a gas
Increases
Decreases
Remains unchanged
First decreases and then increases
The first law of thermodynamics is a statement of conservation of
Heat
Momentum
Energy
Work
The chemical potential for a pure substance is __________ its partial molal free energy.
More than
Less than
Equal to
Not related to
With increase in temperature, the internal energy of a substance
Increases
Decreases
Remains unchanged
May increase or decrease; depends on the substance
High __________ is an undesirable property for a good refrigerant.
Specific heat
Latent heat of vaporisation
Viscosity
Specific vapor volume
The equilibrium constant for a chemical reaction at two different temperatures is given by
Kp2/Kp1 = - (ΔH/R) (1/T2 - 1/T1)
Kp2/Kp1 = (ΔH/R) (1/T2 - 1/T1)
Kp2/Kp1 = ΔH (1/T2 - 1/T1)
Kp2/Kp1 = - (1/R) (1/T2 - 1/T1)
The unit of fugacity is the same as that of the
Pressure
Temperature
Volume
Molar concentration
Pick out the correct statement.
If an insoluble gas is passed through a volatile liquid placed in a perfectly insulated container, the temperature of the liquid will increase
A process is irreversible as long as Δ S for the system is greater than zero
The mechanical work done by a system is always equal to∫P.dV
The heat of formation of a compound is defined as the heat of reaction leading to the formation of the compound from its reactants
Heat of formation of an element in its standard state is
0
< 0
> 0
A function of pressure
The change in Gibbs free energy for vaporisation of a pure substance is
Positive
Negative
Zero
May be positive or negative
The equation, Cp - Cv = R, is true for __________ gas.
No
Any real
Only ideal
Both (B) and (C)
Third law of thermodynamics is helpful in
Prediction of the extent of a chemical reaction
Calculating absolute entropies of substances at different temperature
Evaluating entropy changes of chemical reaction
Both (B) and (C)
The point at which all the three (solid, liquid and gas) phases co-exist, is known as the __________ point.
Freezing
Triple
Boiling
Boyle
A two stage compressor is used to compress an ideal gas. The gas is cooled to the initial temperature after each stage. The intermediate pressure for the minimum total work requirement should be equal to the __________ mean of P1 and P2. (where, P1 and P2 are initial and final pressures respectively)
Logarithmic
Arithmetic
Geometric
Harmonic
The internal energy of an incompressible fluid depends upon its
Pressure
Temperature
Both (A) & (B)
Neither (A) nor (B)
The Carnot co-efficient of performance (COP) of a domestic air conditioner compared to a household refrigerator is
Less
More
Same
Dependent on climatic conditions
Heat pump
Accomplishes only space heating in winter
Accomplishes only space cooling in summer
Accomplishes both (A) and (B)
Works on Carnot cycle
The relation connecting the fugacities of various components in a solution with one another and to composition at constant temperature and pressure is called the __________ equation.
Gibbs-Duhem
Van Laar
Gibbs-Helmholtz
Margules
At normal boiling point, molar entropy of vaporisation is __________ Joule/K°.mole.
72
92
142
192
Which of the following diagrams does not represent an Otto cycle?
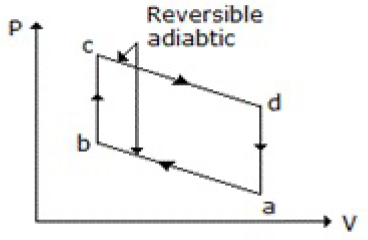
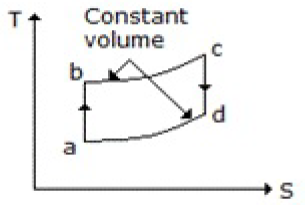
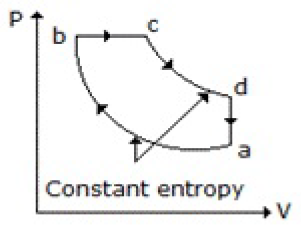
None of these
Which of the following is true for Virial equation of state?
Virial co-efficients are universal constants
Virial co-efficients 'B' represents three body interactions
Virial co-efficients are function of temperature only
For some gases, Virial equations and ideal gas equations are the same
Third law of thermodynamics is concerned with the
Value of absolute entropy
Energy transfer
Direction of energy transfer
None of these
Near their critical temperatures, all gases occupy volumes __________ that of the ideal gas.
Less than
Same as
More than
Half
Efficiency of a heat engine working on Carnot cycle between two temperature levels depends upon the
Two temperatures only
Pressure of working fluid
Mass of the working fluid
Mass and pressure both of the working fluid
Fugacity of a component in an ideal gas mixture is equal to the partial pressure of that component in the mixture. The fugacity of each component in a stable homogeneous solution at constant pressure and temperature __________ as its mole fraction increases.
Decreases
Decreases exponentially
Increases
Remain constant
Isobaric process means a constant process.
Temperature
Pressure
Volume
Entropy
The main feature of Carnot refrigeration cycle is that, it
Does not need the addition of external work for its functioning
Transfers heat from high temperature to low temperature
Accomplishes the reverse effect of the heat engine
None of these
Efficiency of a Carnot engine working between temperatures T1 and T2 (T1 < T) is
(T2 - T1)/T2
(T2 - T1)/T1
(T1 - T2)/T2
(T1 - T2)/T1
