For a constant volume process __________ by the system is used only to increase the internal energy.
Heat absorbed
Work done
Both (A) & (B)
Neither (A) nor (B)
Correct Answer :
A. Heat absorbed
Related Questions
All gases above its inversion temperature, in a throttling process will show
A heating effect
No change in temperature
A cooling effect
Either (A) or (C)
Co-efficient of performance for a reversed Carnot cycle working between temperatures T1 and T2 (T1 > T2) is
T2/(T1 - T2)
T1/(T1 - T2)
(T1 - T2)/T1
(T1 - T2)/T2
For the gaseous phase chemical reaction, C2H4(g) + H2O(g) ↔ C2H5OH(g), the equilibrium conversion does not depend on the
Steam to ethylene ratio
Temperature
Pressure
None of these
Fugacity is a measure of the
Escaping tendencies of the same substance in different phases of a system
Relative volatility of a mixture of two miscible liquids
Behaviour of ideal gases
None of these
What is the value of maximum COP in case of absorption refrigeration, if refrigeration provided is at temperature, TR (where, T1 and T2 are source & surrounding temperatures respectively.)?
TR/(T2 - TR) × (T1 - T2)/T1
TR/(T2 - TR) × T1/(T1 - T2)
TR/(T1 - TR) × (T1 - T2)/T1
None of these
Forward reaction will be favoured for the exothermic reaction, represented by CO + H2O CO2 + H2, by
Low temperature and high pressure
Low temperature and low pressure
High temperature and high pressure
High temperature and low pressure
In an ideal solution, the activity of a component equals its
Mole fraction
Fugacity at the same temperature and pressure
Partial pressure
None of these
In polytropic process (PVn = constant), if n = 1; it means a/an __________ process.
Adiabatic
Reversible
Isothermal
None of these
Pick out the undesirable property for a good refrigerant.
High thermal conductivity
Low freezing point
Large latent heat of vaporisation
High viscosity
If an ideal solution is formed by mixing two pure liquids in any proportion, then the __________ of mixing is zero
Enthalpy
Volume
Both 'a' & 'b'
Neither 'a' nor 'b'
Free energy change at equilibrium is
Zero
Positive
Negative
Indeterminate
A Carnot cycle consists of the following steps:
Two isothermal and two isentropic
Two isobaric and two isothermal
Two isochoric and two isobaric
Two isothermals and two isochoric
Consider the process A & B shown in the figure given below: In this case, it is possible that
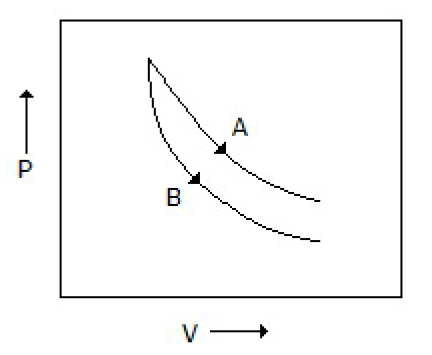
Both the processes are adiabatic
Both the processes are isothermal
Process A is isothermal while B is adiabatic
Process A is adiabatic while B is isothermal
Degree of freedom of a system consisting of a gaseous mixture of H2 and NH3 will be
0
1
2
3
One ton of refrigeration is defined as the heat rate corresponding to melting of one ton of ice in one
Hour
Day
Minute
Second
Internal energy change of a system over one complete cycle in a cyclic process is
Zero
+ve
-ve
Dependent on the path
Grams of butane (C4H10) formed by the liquefaction of 448 litres of the gas (measured at (STP) would be
580
640
1160
Data insufficient; can't be computed
The equilibrium constant for a chemical reaction at two different temperatures is given by
Kp2/Kp1 = - (ΔH/R) (1/T2 - 1/T1)
Kp2/Kp1 = (ΔH/R) (1/T2 - 1/T1)
Kp2/Kp1 = ΔH (1/T2 - 1/T1)
Kp2/Kp1 = - (1/R) (1/T2 - 1/T1)
The reaction A (l) → R(g) is allowed to reach equilibrium conditions in an autoclave. At equilibrium, there are two phases, one a pure liquid phase of A and the other a vapor phase of A, R and S. Initially A alone is present. The numbers of degrees of freedom are:
1
2
3
0
Which of the following diagrams does not represent an Otto cycle?
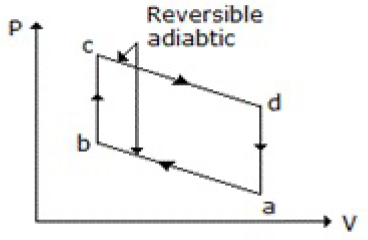
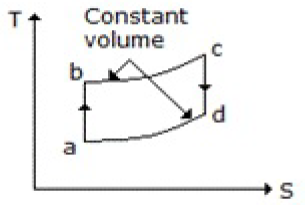
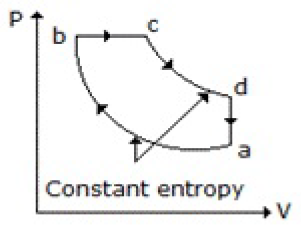
None of these
The adiabatic throttling process of a perfect gas is one of constant enthalpy
In which there is a temperature drop
Which is exemplified by a non-steady flow expansion
Which can be performed in a pipe with a constriction
In which there is an increase in temperature
Solubility of a substance which dissolves with an increase in volume and liberation of heat will be favoured by the
Low pressure and high temperature
Low pressure and low temperature
High pressure and low temperature
High pressure and high temperature
The value of gas constant 'R' is
1.987 cal/gm mole °K
1.987 BTU/lb. mole °R
Both (A) and (B)
Neither (A) nor (B)
__________ functions are exemplified by heat and work.
Path
Point
State
None of these
The number of degree of freedom for an Azeotropic mixture of ethanol and water in vapourliquid equilibrium, is
3
1
2
0
In jet refrigerators, the refrigerating fluid is practically always
Water
Ammonia
Freon
Brine
Which of the following is not affected by temperature changes?
Fugacity
Activity co-efficient
Free energy
None of these
For a stable phase at constant pressure and temperature, the fugacity of each component in a binary system __________ as its mole fraction increases.
Decreases
Increases
Remain same
Decreases linearly
Dry ice is
Moisture free ice
Solid helium
Solid carbon dioxide
None of these
For an irreversible process involving only pressure-volume work
(dF)T, p <0
(dF)T, p = 0
(dF)T, p > 0
(dA)T, v >0
