Gibbs free energy of a pure fluid approaches __________ as the pressure tends to zero at constant temperature.
Infinity
Minus infinity
Zero
None of these
Correct Answer :
B. Minus infinity
Related Questions
The expression for the work done for a reversible polytropic process can be used to obtain the expression for work done for all processes, except reversible __________ process.
Isobaric
Isothermal
Adiabatic
None of these
Air enters an adiabatic compressor at 300K. The exit temperature for a compression ratio of 3, assuming air to be an ideal gas (Y = Cp/Cv = 7/5) and the process to be reversible, is
300 × (32/7)
300 × (33/5)
300 × (333/7)
300 × (35/7)
For a constant volume process __________ by the system is used only to increase the internal energy.
Heat absorbed
Work done
Both (A) & (B)
Neither (A) nor (B)
Compound having large heat of formation is
More stable
Less stable
Not at all stable (like nascent O2)
Either more or less stable; depends on the compound
Degree of freedom of a system consisting of a gaseous mixture of H2 and NH3 will be
0
1
2
3
Internal energy of an ideal gas
Increases with increase in pressure
Decreases with increase in temperature
Is independent of temperature
None of these
The heat capacities for the ideal gas state depend upon the
Pressure
Temperature
Both (A) & (B)
Neither (A) nor (B)
The adiabatic throttling process of a perfect gas is one of constant enthalpy
In which there is a temperature drop
Which is exemplified by a non-steady flow expansion
Which can be performed in a pipe with a constriction
In which there is an increase in temperature
Cp of a gas at its critical temperature and pressure
Becomes zero
Becomes infinity
Equals 1 kcal/kmol °K
Equals 0.24 kcal/kmol °K
The four properties of a system viz. P, V, T, S are related by __________ equation.
Gibbs-Duhem
Gibbs-Helmholtz
Maxwell's
None of these
Isotherm on an enthalpy-concentration diagram, for an ideal solution will be a
Straight line
Sine curve
Parabola
Hyperbola
Air-refrigeration cycle
Is the most efficient of all refrigeration cycles
Has very low efficiency
Requires relatively large quantities of air to achieve a significant amount of refrigeration
Both (B) and (C)
In the reaction, represented by, 2SO2 + O2 2SO3; ΔH = - 42 kcal; the forward reaction will be favoured by
Low temperature
High pressure
Both (A) and (B)
Neither (A) nor (B)
Near their critical temperatures, all gases occupy volumes __________ that of the ideal gas.
Less than
Same as
More than
Half
Pick out the correct statement.
Compression ratio of an Otto engine is comparatively higher than a diesel engine
Efficiency of an Otto engine is higher than that of a diesel engine for the same compression ratio
Otto engine efficiency decreases with the rise in compression ratio, due to decrease in work produced per quantity of heat
Diesel engine normally operates at lower compression ratio than an Otto engine for an equal output of work
For a cyclic process, a fixed ratio between heat and work
Always exists
May exist
Never exists
Is difficult to predict
Compressibility factor for almost all the gases are approximately same at the same
Pressure and temperature
Reduced pressure and reduced temperature
Critical pressure and critical temperature
None of these
The expression for entropy change, ΔS = n Cp . ln (T2/T1), is valid for the __________ of a substance.
Simultaneous pressure & temperature change
Heating
Cooling
Both (B) and (C)
In which of the following reaction equilibrium, the value of equilibrium constant Kp will be more than is Kc?
2HI H2 + I2
N2O4 2NO2
2SO2 + O2 2SO3
None of these
In any spontaneous process, the __________ free energy decreases.
Helmholtz
Gibbs
Both a & b
Neither 'a' nor 'b'
As the temperature is lowered towards the absolute zero, the value of ∂(ΔF)/∂T, then approaches
Unity
Zero
That of the heat of reaction
Infinity
Number of degrees of freedom for a three phase system in equilibrium comprising of three nonreacting chemical species is
2
0
1
3
Which of the following is an undesirable characteristic of a refrigerant?
It should be non-explosive
It should have a sub-atmospheric vapor pressure at the temperature in refrigerator coils
Its vapor pressure at the condenser temperature should be very high
None of these
The principle applied in liquefaction of gases is
Adiabatic expansion
Joule-Thomson effect
Both (A) and (B)
Neither (A) nor (B)
Which of the following is not a common refrigerant?
Freon-12
Ethylene
Ammonia
Carbon dioxide
An ideal monatomic gas is taken round the cycle ABCDA as shown below in the P-V diagram. The work done during the cycle is
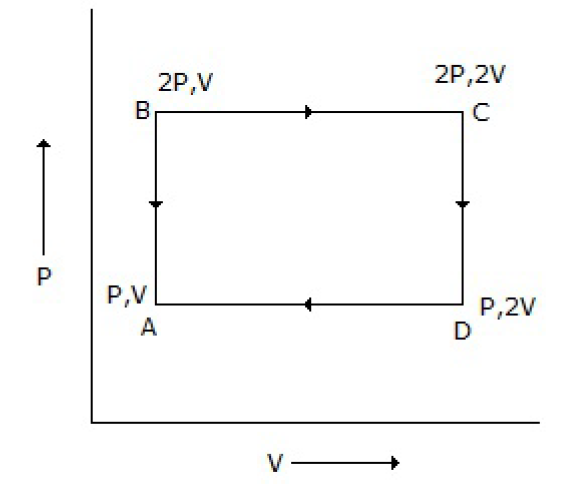
PV
2PV
PV/2
0
__________ does not change during phase transformation processes like sublimation, melting & vaporisation.
Entropy
Gibbs free energy
Internal energy
All (A), (B) & (C)
One mole of nitrogen at 8 bar and 600 K is contained in a piston-cylinder arrangement. It is brought to 1 bar isothermally against a resisting pressure of 1 bar. The work done (in Joules) by the gas is
30554
10373
4988.4
4364.9
Which of the following is an extensive property of a system?
Heat capacity
Molal heat capacity
Pressure
Concentration
Filling of gas from a high pressure cylinder into small bottles is an example of a/an __________ process.
Equilibrium
Adiabatic
Steady
Unsteady
