The cooling rate required to freeze 1 ton of water at 0°C into ice at 0°C in 24 hours is __________ BTU/hr.
100
200
1000
2000
Correct Answer :
B. 200
Related Questions
A form of stress corrosion failure termed as 'season cracking' is generally observed in
Thermosetting polymers
High carbon steels
Brasses
Borosilicate glasses
Maximum amount of thermal radiation is emitted at all wavelengths at any specified temperature by a/an __________ body.
Grey
Black
Opaque
White
Theoretical volume of oxygen required for complete combustion of 1Nm3 of acetylene, in oxyacetylene welding is __________ Nm3.
0.5
1
1/3
2.5
A furnace is made of a refractory brick wall of thickness 0.5 metre and thermal conductivity 0.7 W/m.°K For the same temperature drop and heat loss, this refractory wall can be replaced by a layer of diatomaceous earth of thermal conductivity 0.14 W/m.K and thickness __________ metre.
0.01
0.1
0.25
0.5
Shaft/rotor speed is most accurately measured by a
Frequency counter
Tachometer
Strobometer
Speedometer
Window panes of aeroplanes are normally made of
Perspex (PMMA)
Teflon (PTFE)
Bakelite (phenol formaldehyde)
Polystyrene
__________ test can be termed as the semidestructive test.
Impact
Torsion
Hardness
Charpy
Which of the following test is used for distinguishing among dry oils, semi-drying oils and non drying oils?
Elaiden test
Reichert-Meissl value test
Hunter value test
Iodine value test
__________ is the hardest oxide and is hence used where high wear resistance at high temperature is required.
Beryllium
Zirconia
Alumina
Magnesia
Work study deals with the __________ study.
Time
Motion
Both (A) & (B)
Neither (A) nor (B)
In the Bayer's process, bauxite is digested under pressure using
H2SO4
NaOH
NH3
HCl
Which of the following is an example of stress corrosion?
Season cracking of brass
Caustic embrittlement of steel
Both (A) & (B)
Neither (A) nor (B)
Energy of the sun arises mainly from __________ reactions.
Fusion
Fission
Combustion
None of these
The materials which fracture even at small strains are termed as brittle, while those materials which exhibit an appreciable deformation before failure are termed as
Rigid
Tough
Ductile
Plastic
Isotropic materials have the same __________ in all directions.
Induced stresses
Density
Elastic properties
Thermal properties
Which of the following if present in iron ore, enhances its value?
Alumina
Alkali oxide
Lime & Magnesia
None of these
The difference between gross & net calorific values of fuel is due to the
Sensible heat carried away by the flue gases
Heat carried away by the steam from the moisture content of the fuel
Heat lost by radiation
Heat carried away by steam from the combustion of hydrogen in the fuel
Melting point & boiling points of liquid oxygen are respectively - 218.8°C & - 183°C, while the same for liquid nitrogen is - 210°C & - 195.8°C respectively. The difference in melting points of liquid oxygen & liquid nitrogen is not equal to 8.8
°C
°F
°K
°R
Maximum heat dissipation occurs from a steel wire (k = 0.5 W/m. k) of 15 mm diameter exposed to air (h = 20W/m2 .k), when the insulation thickness is __________ mm.
15
25
10
30
A semi-conductor is damaged & behaves like a conductor, on passing a strong electric current through it, because it heats up the crystals & breaks the covalent bonds resulting in __________ electrons.
Lack of free
Excess of
Decrease in
None of these
Which of the following can be manufactured using powder metallurgy techniques?
Brake linings
Bearings
Carbide tool tips
All 'a', 'b' and 'c'
For a first order chemical reaction, the concentration of the reactant decreases __________ with time.
Linearly
Exponentially
Logarithmically
Inversely
Square steel key is normally strong in failure by shear & crushing. Keys are normally made of __________ steel bars.
Hot rolled mild
Cold rolled mild
Forged
Machinable stainless
Minimum safe distance between two liquid fuel storage tanks is equal to (where, H = height of the tank)
H
H/2
H/4
H/6
__________ test is the appropriate test to determine whether a material is ductile or brittle.
Impact
Cupping
Hardness
Tensile
Which of the following phenomenon/ phenomena is/are diffusion controlled?
Dislocation climb
Cross-slip
Twinning
Recrystallisation
Which of the following has the highest modulus of elasticity (about 7 × 106 kg/cm2)?
High speed steel
Stainless steel
Tungsten carbide
Superalloys
Which of the following mainly decides the current to be employed is arc welding?
Electrode size
Plate thickness
Voltage across the arc
Welded portion length
The phenomenon occurring during explosion of a hydrogen bomb is
Nuclear fission
Nuclear fusion
A combination of both nuclear fission & fusion
None of these
Maraging steels derive their strength from the following mechanism:
A fine, highly dislocated and strong martensite
Fine dispersions of inter-metallic of Fe, Ni, Ti etc
Fine dispersions of alloy carbides in a ferrite matrix
Fine dispersions of 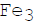 C nucleated on dislocations in austenite
C nucleated on dislocations in austenite
