Internal energy of a gas obeying Van-Der- Waals equation of state, [p + (a/v2)] (V - b) = RT, depends upon its
Pressure & temperature
Pressure & specific volume
Temperature & specific volume
Temperature only
Correct Answer :
A. Pressure & temperature
Related Questions
The heat released by cooling one mole of copper from 400 K to room temperature (300 K) is (assume : Cp of copper is 23 J K-1mole-1)
2300 J
4600 J
230 J
2.3 × 106 J
Friction factor for fluid flow in pipe does not depend upon the
Pipe length
Pipe roughness
Fluid density & viscosity
Mass flow rate of fluid
The specific gravity of coal depends mainly on its __________ content.
Carbon
Volatile matter
Ash
Moisture
Which of the following materials does not form adherent oxide film on surface?
Copper
Nickel
Aluminium
Gold & silver
Which of the following metals is the most prone to work hardening?
Brass
Aluminium
Copper
Lead
Addition of __________ to steel does not help in improving its machinability.
Sulphur
Silicon
Lead
Phosphorous
For infinite parallel planes having emissivities ε1 & ε2, the interchange factor for radiation from surface 1 to surface 2 is given by
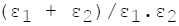
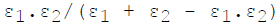
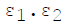
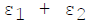
Minimum number of members required to form a Public Limited Joint Stock Com-pany is
7
10
12
17
Hot extrusion process is not used for making
Cast iron pipe for domestic water supply
Aluminium curtain rods
Stainless steel tubes used in furnitures
Any of these
Midrex process of sponge iron production uses reformed natural gas as the reducing agent, which uses iron ore in the form of
Lumps
Pellets
Briquettes
Sinter
Co-efficient of performance of a Carnot cycle refrigerator operating between - 23°C and + 27°C is
3
5
0.5
1.5
Annealing of white cast iron produces __________ iron.
Grey
Nodular
Malleable
Spheroidal
Optical activity is a/an __________ property.
Additive
Constitutive
Both (A) & (B)
Neither (A) nor (B)
Normalising of a casting does not
Induce stresses in it
Refine its grain structure
Reduce segregation
Improve its mechanical properties
The temperature at which the magnetic property of iron disappears (i.e., it becomes non-magnetic) and its electrical conductivity & specific heat also changes, is called the 'Curie point', which is __________ °C.
768
908
1400
1539
In case of a, centrifugal pump, the ratio h1/h2 is termed as the __________ efficiency (where, h1 = actual measured head & h2 = head imparted to the fluid by impeller).
Mechanical
Overall
Volumetric
Impeller
In a furnace with heating element temperature at 1700°C, the dominant mechanism of heat transfer will be
Conduction
Radiation
Natural convection
Forced convection
Electrolytic reduction cell used for conversion of calcined Al2O3 to Al is a carbon lined furnace operating at 800-900 °C. The purpose of electric current supplied to the furnace is to
Achieve very high purity of aluminium (99.9%)
Keep the electrolyte in liquid condition by the generation of heat
Electrolytically dissociate alumina
Both (B) & (C)
Materials having resistivity ranging from 1 to 100 ohm. cm is termed as
Conductor
Insulator
Semi-conductor
None of these
Which one of the following is incombustible?
H2
CCl4
C2H2
S
__________ rubber is generally used for making 'O' rings used for vacuum sealings.
Natural
Neoprene
Butadiene
Nitrile
Which of the following is not the function of a volute casing provided in a centrifugal pump?
To reduce the head loss in discharge
To increase the pump efficiency
To collect liquid from the periphery of the impeller and to transmit it to the delivery pipe at constant velocity
To increase the pump discharge rate
During decarburising of a plain carbon steel, the thickness of ferrite layer growth is proportional to
Time
Square root of time
Square of time
Cube of time
The taper provided on pattern for its easy & clean withdrawal from the mould is termed as the __________ allowance.
Casting
Pattern
Draft
Distortion
Powder metallurgy technique is used in the production of __________ tools.
Tungsten carbide
High carbon steel
High speed steel
Drilling
Increase in temperature, in general results in the
Decrease in the viscosities of both liquids & gases
Increase in the viscosities of both liquids & gases
Increase in the viscosity of liquids and decrease in that of gases
Decrease in the viscosity of liquids and increase in that of gases
Cold chisels hammers are made of
High speed steel
High carbon steel
Forged steel
Mild steel
Property responsible for the talcum powder to cling to the skin is the
Capillary action
Adhesion
Cohesion
Surface tension
In the Bayer's process, bauxite is digested under pressure using
H2SO4
NaOH
NH3
HCl
The starting of a car takes time in winter, because the
Octane number of fuel is decreased
Fuel supply for ignition is not sufficient
Vaporisation of the fuel is decreased
Pour point of fuel decreases
