Compressibility factor of a gas is
Not a function of its pressure
Not a function of its nature
Not a function of its temperature
Unity, if it follows PV = nRT
Correct Answer :
A. Not a function of its pressure
Related Questions
Third law of thermodynamics is concerned with the
Value of absolute entropy
Energy transfer
Direction of energy transfer
None of these
Which of the following has the minimum value of COP for a given refrigeration effect?
Reverse Carnot cycle
Ordinary vapour-compression cycle
Vapour-compression process with a reversible expansion engine
Air refrigeration cycle
The effect of changing the evaporator temperature on COP as compared to that of changing the condenser temperature (in vapour compression refrigeration system) is
Less pronounced
More pronounced
Equal
Data insufficient, can't be predicted
The third law of thermodynamics states that the
Heat capacity of a crystalline solid is zero at absolute zero temperature
Heat transfer from low temperature to high temperature source is not possible without external work
Gases having same reduced properties behaves similarly
None of these
Which of the following is not an equation of state?
Bertholet equation
Clausius-Clapeyron equation
Beattie-Bridgeman equation
None of these
Fugacity and pressure are numerically not equal for the gases
At low temperature and high pressure
At standard state
Both (A) and (B)
In ideal state
The entropy change in a reversible isothermal process, when an ideal gas expands to four times its initial volume is
R loge 4
R log10 4
Cv log10 4
Cv loge 4
Specific volume of an ideal gas is
Equal to its density
The reciprocal of its density
Proportional to pressure
None of these
What is the degree of freedom for a system comprising liquid water equilibrium with its vapour?
0
1
2
3
Which of the following is not an extensive property?
Free energy
Entropy
Refractive index
None of these
Consider the reaction, C + O2 CO2; ΔH = - 94 kcal. What will be the value of ΔH for the reaction CO2 → C + O2?
-94 kcal
+94 kcal
> 94 kcal
< -94 kcal
A thermodynamic system is taken from state A to B along ACB and is brought back to A along BDA as shown below in the P-V diagram. The net work done during the complete cycle is given by the area covered by
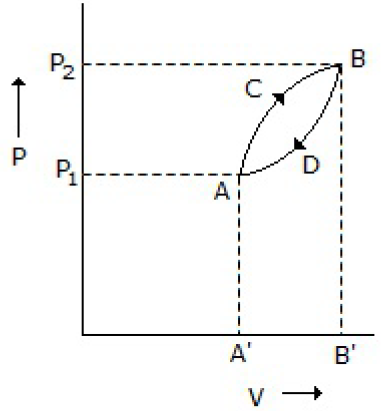
P1ACBP2P1
ACBB1A1A
ACBDA
ADBB1A1A
Solubility of a substance which dissolves with an increase in volume and liberation of heat will be favoured by the
Low pressure and high temperature
Low pressure and low temperature
High pressure and low temperature
High pressure and high temperature
Refrigerants commonly used for domestic refrigerators are
Ethyl chloride or methyl chloride
Freon-12
Propane
NH3 or CO2
Specific __________ does not change during phase change at constant temperature and pressure.
Entropy
Gibbs energy
Internal energy
Enthalpy
In any spontaneous process,
Only F decreases
Only A decreases
Both F and A decreases
Both F and A increase
Heat of formation of an element in its standard state is
0
< 0
> 0
A function of pressure
The freezing point of a liquid decreases when the pressure is increased, if the liquid __________ while freezing.
Contracts
Expands
Does not change in volume
Either (A), (B) or (C)
For an exothermic reaction
Only enthalpy change (ΔH) is negative
Only internal energy change (ΔE) is negative
Both ΔH and ΔE are negative
Enthalpy change is zero
Which of the following is a widely used refrigerant in vapour compression refrigeration system (using large centrifugal compressor)?
Freon
Liquid sulphur dioxide
Methyl chloride
Ammonia
Gibbs free energy of a pure fluid approaches __________ as the pressure tends to zero at constant temperature.
Infinity
Minus infinity
Zero
None of these
When a gas is subjected to adiabatic expansion, it gets cooled due to
Decrease in velocity
Decrease in temperature
Decrease in kinetic energy
Energy spent in doing work
__________ increases with increase in pressure.
The melting point of wax
The boiling point of a liquid
Both (A) and (B)
Neither (A) nor (B)
The equation relating E, P, V and T which is true for all substances under all conditions is given by (∂E/∂V)T = T(∂P/∂T)H - P. This equation is called the
Maxwell's equation
Thermodynamic equation of state
Equation of state
Redlich-Kwong equation of state
The free energy change for a chemical reaction is given by (where, K = equilibrium constant)
RT ln K
-RT ln K
-R ln K
T ln K
The adiabatic throttling process of a perfect gas is one of constant enthalpy
In which there is a temperature drop
Which is exemplified by a non-steady flow expansion
Which can be performed in a pipe with a constriction
In which there is an increase in temperature
The rate at which a substance reacts is proportional to its active mass and the rate of a chemical reaction is proportional to the product of active masses of the reacting substances. This is the
Lewis-Randall rule
Statement of Van't Hoff Equation
Le-Chatelier's principle
None of these
For any system, what is the minimum number of degrees of freedom?
0
1
2
3
The acentric factor of a materical, 'ω', is defined as ω = -log10(Prsat)Tr-1 = 0.7, where, Prsat = reduced vapor pressure, Tr = reduced temperature. The value of acentric factor is always
> 2
< 1
> 1
< 3
Change of heat content when one mole of compound is burnt in oxygen at constant pressure is called the
Calorific value
Heat of reaction
Heat of combustion
Heat of formation
