A refrigerator may be termed as a
Heat pump
Heat engine
Carnot engine
None of these
Correct Answer :
A. Heat pump
Related Questions
The equation, (d loge PA/d loge xA) = (d loge PA/d loge xB) applicable to a binary solution of components. A and B in equilibrium with their vapors at constant temperature and pressure is called the __________ equation.
Van Laar
Margules
Gibbs-Duhem
Gibbs-Duhem-Margules
Which one is true for a throttling process?
A gas may have more than one inversion temperatures
The inversion temperature is different for different gases
The inversion temperature is same for all gases
The inversion temperature is the temperature at which Joule-Thomson co-efficient is infinity
The melting point of paraffin wax (which contracts on solidification) __________ with pressure rise.
Increases
Decreases
Remains unchanged
Decreases linearly
The equation relating E, P, V and T which is true for all substances under all conditions is given by (∂E/∂V)T = T(∂P/∂T)H - P. This equation is called the
Maxwell's equation
Thermodynamic equation of state
Equation of state
Redlich-Kwong equation of state
Solubility of a substance which dissolves with an increase in volume and liberation of heat will be favoured by the
Low pressure and high temperature
Low pressure and low temperature
High pressure and low temperature
High pressure and high temperature
Specific __________ does not change during phase change at constant temperature and pressure.
Entropy
Gibbs energy
Internal energy
Enthalpy
The expression for entropy change given by, ΔS = nR ln (V2/V1) + nCv ln (T2/T1) is valid for
Reversible isothermal volume change
Heating of a substance
Cooling of a substance
Simultaneous heating and expansion of an ideal gas
A change in state involving a decrease in entropy can be spontaneous, only if
It is exothermic
It is isenthalpic
It takes place isothermally
It takes place at constant volume
Melting of wax is accompanied with __________ in entropy.
Increase
Decrease
No change
None of these
What happens in a reversible adiabatic compression?
Heating occurs
Cooling occurs
Pressure is constant
Temperature is constant
Entropy is a measure of the __________ of a system.
Disorder
Orderly behaviour
Temperature changes only
None of these
For an ideal gas, the internal energy depends upon its __________ only.
Molecular size
Temperature
Volume
Pressure
An irreversible process
Is the analog of linear frictionless motion in machines
Is an idealised visualisation of behaviour of a system
Yields the maximum amount of work
Yields an amount of work less than that of a reversible process
PVγ = Constant (where, γ = Cp/Cv) is valid for a/an __________ process.
Isothermal
Isentropic
Isobaric
Adiabatic
The freezing point of a liquid decreases when the pressure is increased, if the liquid __________ while freezing.
Contracts
Expands
Does not change in volume
Either (A), (B) or (C)
In jet refrigerators, the refrigerating fluid is practically always
Water
Ammonia
Freon
Brine
Which of the following diagrams does not represent an Otto cycle?
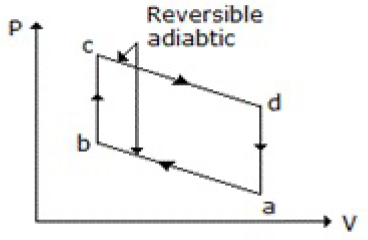
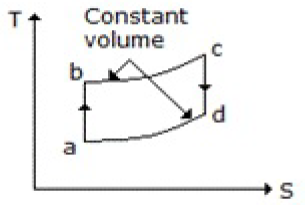
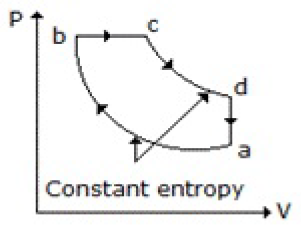
None of these
Which of the following is not affected by temperature changes?
Fugacity
Activity co-efficient
Free energy
None of these
Entropy change of the reaction, H2O (liquid) → H2O (gas), is termed as the enthalpy of
Solution
Vaporisation
Formation
Sublimation
Internal energy of an ideal gas
Increases with increase in pressure
Decreases with increase in temperature
Is independent of temperature
None of these
The kinetic energy of gas molecule is zero at
0°C
273°C
100°C
-273°C
To obtain integrated form of Clausius-Clapeyron equation, ln (P2/P1) = (ΔHV/R) (1/T1 - 1/T2) from the exact Clapeyron equation, it is assumed that the
Volume of the liquid phase is negligible compared to that of vapour phase
Vapour phase behaves as an ideal gas
Heat of vaporisation is independent of temperature
All (A), (B) & (C)
Those solutions in which there is no volume change upon mixing the components in the liquid state and which, when diluted do not undergo any heat change (i.e. heat of dilution is zero), are called __________ solutions.
Ideal
Real
Isotonic
None of these
The equation DU = Tds - PdV is applicable to infinitesimal changes occuring in
An open system of constant composition
A closed system of constant composition
An open system with changes in composition
A closed system with changes in composition
For a real gas, the chemical potential is given by
RT d ln P
RT d ln f
R d ln f
None of these
A solid is transformed into vapour without going to the liquid phase at
Triple point
Boiling point
Below triple point
Always
The chemical potential for a pure substance is __________ its partial molal free energy.
More than
Less than
Equal to
Not related to
The compressibility factor for an ideal gas is 1. Its value for any other real gas is
1
< 1
> 1
Either (B) or (C), depends on the nature of the gas
In the equation, PVn = constant, if the value of n = 1, then it represents a reversible __________ process.
Isothermal
Isobaric
Polytropic
Adiabatic
Co-efficient of performance for a reversed Carnot cycle working between temperatures T1 and T2 (T1 > T2) is
T2/(T1 - T2)
T1/(T1 - T2)
(T1 - T2)/T1
(T1 - T2)/T2
